Global Clinical Trials Market Size To Grow USD 93.42 Billion by 2032 | CAGR of 6.4%
Category: HealthcareGlobal Clinical Trials Market to Surpass $93.42 Billion by 2032
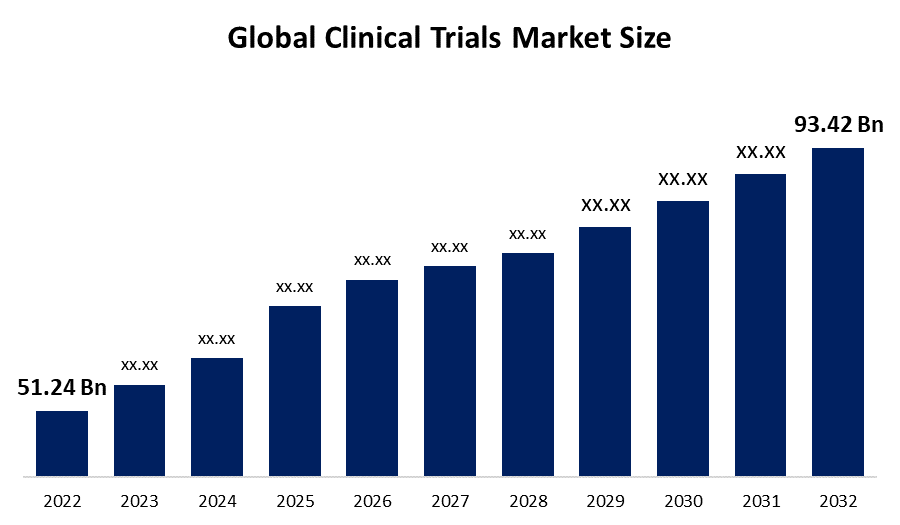
According to a research report published by Spherical Insights & Consulting, the Global Clinical Trials Market Size is to grow from USD 51.24 billion in 2022 to USD 93.42 billion by 2032, at a Compound Annual Growth Rate (CAGR) of 7.04% during the projected period. The increasing utilization of clinical research in various indications and therapies such as obesity, cardiovascular, diabetes, oncology, pain management, CNS condition, autoimmune, inflammation, and others in several applications including vaccines, cell & gene therapy, small molecules, and others is expected to boost the demand for the clinical trials market during the forecast period.
Get more details on this report -
Browse key industry insights spread across 215 pages with 124 market data tables and figures & charts from the report on " Global Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Phase (Phase 0, Phase I, Phase II, Phase III, Phase IV), By Indication, By Therapy, By Service Type, By Application (Vaccine, Cell & Gene Therapy, Small Molecules, Other Applications), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2022 – 2032 ” Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/clinical-trials-market
Clinical trials are a type of research in which new tests and treatments are investigated and the influence on human health outcomes is assessed. Each licensed medical practice can cost billions of dollars in clinical trials. Clinical trials are typically sponsored by the government or a pharmaceutical, biotechnology, or medical device company. However, just 10% of all pharmaceuticals that begin in human clinical trials are approved for use. The increased demand for breakthrough pharmacological solutions to treat chronic conditions such as cancer, respiratory difficulties, heart disease, Type 2 diabetes, and others places immense strain on the healthcare system. The bulk of pharmaceutical, biopharmaceutical, and healthcare device companies continue to invest heavily in the development of novel medicines and technologies. Furthermore, this market is expected to grow as a consequence of factors such as clinical studies expanding their reach, technological developments, and a corresponding growth in the need for CROs to execute research activities.
The Phase III segment is dominating the market with the largest revenue share over the forecast period.
On the basis of phase, the global clinical trials market is segmented into Phase 0, Phase I, Phase II, Phase III, and Phase IV. Owing to the high costs involved with Phase III trials being the most expensive and including many subjects, the Phase III category is dominating the market with an overall revenue share of 48.6% throughout the projection period. likewise, Phase III necessitates a larger number of patients and, in many cases, a longer treatment term.
The autoimmune & inflammation segment is witnessing significant CAGR growth over the forecast period.
On the basis of indication, the global clinical trials Market is segmented into obesity, cardiovascular, diabetes, oncology, pain management, CNS condition, autoimmune, inflammation, and others. Among these, the autoimmune & inflammation sector is expected to increase at a substantial CAGR during the projected period, owing to a significant number of clinical trials on autoimmune/inflammation conducted globally.
The oncology segment is witnessing the highest growth rate over the forecast period.
On the basis of therapy, the global clinical trials market is segmented into oncology, infectious diseases, cardiology, neurology, women's health, genetic diseases, immunology, and others. Among these, the oncology segment is witnessing the highest growth rate over the forecast period. The oncology segment is further sub-segmented as blood cancer, solid tumors, and others.
The vaccines segment accounted for the largest revenue share of more than 57.2% over the forecast period.
On the basis of application, the global clinical trials market is segmented into vaccines, cell & gene therapy, small molecules, and others. Among these, the vaccines segment is dominating the market with the largest revenue share of 57.2% over the forecast period. Because the number of people recruited in vaccine clinical trials has been increasing over time, and because the number of participants is connected to price, clinical trial expenses may help explain the observed growth in public- and private-sector vaccination pricing.
North America dominates the market with the largest market share over the forecast period.

Get more details on this report -
North America is dominating the market with more than 53.7% market share over the forecast period. This growth has been credited to increasing financing for research and development as well as increased use of innovative technology in clinical trials in this region. Furthermore, North America is home to the world's largest pharmaceutical market, as well as multiple worldwide pharmaceutical and medical device conglomerates such as Pfizer, Inc., AbbVie, Inc., Abbott Laboratories, and Johnson & Johnson.
Major vendors in the Global Clinical Trials Market include Charles River Laboratory, ICON Plc, Wuxi AppTec Inc, SGS SA, Chiltern International Ltd, Eli Lilly and Company, Omnicare, Kendle, LabCorp, IQVIA, Novo Nordisk A/S, Pfizer, Clinipace, Syneos Health, PAREXEL International Corporation, Pharmaceutical Product Development, LLC, The Emmes Company, LLC, and among others.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- On February 2023, Thermo Fisher Scientific Inc.'s PPD clinical research business, a world leader in serving science, has been awarded a five-year contract to provide regulatory affairs support and related services for the Blueprint MedTech (BPMT) program, a new multi-institute/center initiative at the National Institutes of Health (NIH) supporting the development of translational neurological devices. The BPMT initiative is a new NIH incubator with a collaborative reach across 11 NIH institutes, with the purpose of accelerating patient access to safe, effective, cutting-edge medical devices to diagnose and/or treat nervous system illnesses. The BPMT incubator seeks to assist the development of various unique technologies from pharmaceutical, biotechnology, and device firms during each year of the contract.
- In June 2022, Labcorp announced an expansion of CB Trial Laboratory, the central laboratory co-managed by Labcorp Drug Development and BML, a renowned Japanese provider of clinical laboratory testing services, to strengthen its central laboratory presence and drug development capabilities in Japan. Labcorp Drug Development and BML will commence construction on a new laboratory facility in Kawagoe, Saitama, expanding capacity and services for pharmaceutical and biotechnology clients, extending their strategic cooperation that dates back more than a decade.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2032. Spherical Insights has segmented the Global Clinical Trials Market based on the below-mentioned segments:
Clinical Trials Market, By Phase Analysis
- Phase 0
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trials Market, By Indication Analysis
- Obesity
- Cardiovascular
- Diabetes
- Oncology
- Pain Management
- CNS Condition
- Autoimmune
- Inflammation
- Others
Clinical Trials Market, By Therapy Analysis
- Oncology
- Infectious Diseases
- Cardiology
- Neurology
- Women's Health
- Genetic Diseases
- Immunology
- Others
Clinical Trials Market, By Service Type Analysis
- Protocol Designing
- Site Identification
- Patient Recruitment
- Laboratory Services
- Analytical Testing Services
- Clinical Trial Supply & Logistic Services
- Bioanalytical Testing Services
- Clinical Trial Data Management Services
- Medical Device Testing Services
- Others
Clinical Trials Market, By Application Analysis
- Vaccine
- Cell & Gene Therapy
- Small Molecules
- Others
Clinical Trials Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?