Global Cystatin C Assay Market Worth Size To USD 525.9 Million By 2033 | CAGR of 6.87%
Category: HealthcareGlobal Cystatin C Assay Market Size To Worth USD 525.9 Million By 2033
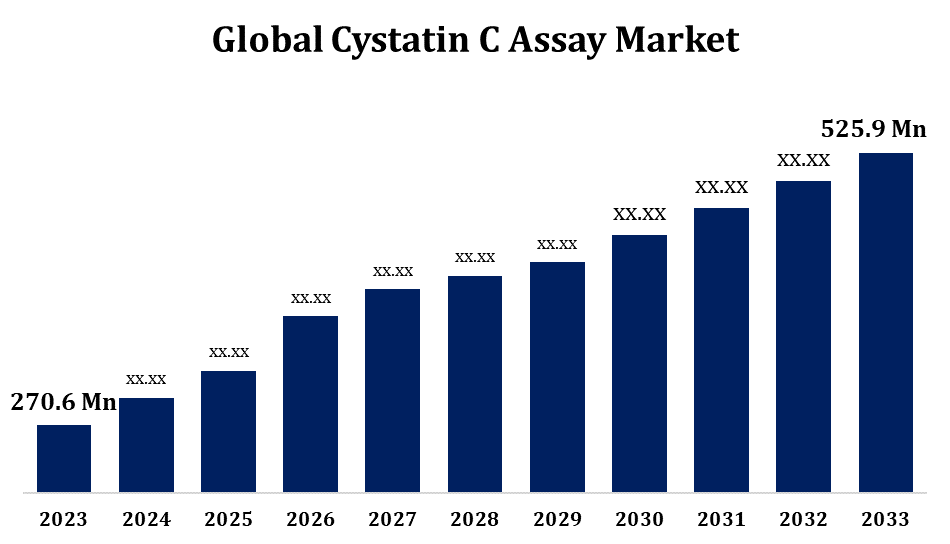
According to a research report published by Spherical Insights & Consulting, the Global Cystatin C Assay Market Size to Gow from USD 270.6 Million in 2023 to USD 525.9 million by 2033, at a Compound Annual Growth Rate (CAGR) of 6.87% during the forecast period.
Get more details on this report -
Browse key industry insights spread across 280 pages with 110 Market data tables and figures & charts from the report on the “Global Cystatin C Assay Market Size, Share, and COVID-19 Impact Analysis, By Product (Kits, Reagents, Analyzers), By Sample Type (Blood, Urine), By Application (Diagnostics, Research), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033.” Get Detailed Report Description Here:https://www.sphericalinsights.com/reports/cystatin-c-assay-market
The cystatin C assay market is expected to grow significantly due to the rising prevalence of kidney diseases, particularly chronic kidney disease (CKD) and acute kidney injury (AKI), as well as the benefits cystatin C provides over traditional creatinine tests, such as greater accuracy regardless of muscle mass variations. Technological developments and the increasing use of point-of-care testing (POCT) improve the efficiency and accessibility of these tests. Despite constraints like as high costs and regulatory hurdles, the market forecast is optimistic, driven by an ageing population, increasing healthcare access, and the incorporation of personalised medical approaches. Efforts to raise awareness and manage expenses will be critical for increased adoption and industry growth.
Cystatin C Assay Market Value Chain Analysis
The value chain analysis of the cystatin C assay market encompasses several essential stages, commencing with the procurement of raw materials and progressing through production, distribution, and eventual end-use application. Raw materials, notably reagents and biological materials, are procured from specialized suppliers and are indispensable for manufacturing high-quality cystatin C assays. The production phase involves the creation of assay kits and instruments, integrating advanced technologies to guarantee precision and dependability. In the end-use application stage, cystatin C assays are employed for diagnosing and monitoring kidney function in patients, with a growing emphasis on personalized medicine and point-of-care testing. Throughout this value chain, factors such as regulatory compliance, cost management, and technological advancements play pivotal roles in influencing market dynamics and ensuring the provision of effective diagnostic solutions to healthcare providers and patients.
Cystatin C Assay Market Opportunity Analysis
The cystatin C assay market has tremendous potential opportunities due to a variety of variables. One of the key opportunities stems from the rising global prevalence of chronic kidney disease (CKD) and acute kidney injury (AKI), which demands more effective diagnostic tools for early diagnosis and treatment. The ageing population exacerbates this demand, as older persons are more prone to renal problems. Technological improvements in diagnostic testing, such as the development of high-throughput and point-of-care testing (POCT) devices, present significant market growth opportunities. These advancements enable faster, more accurate, and convenient testing, which improves patient care and clinical results.
The growing use of point-of-care testing (POCT) in the cystatin C assay market is a major trend driving market expansion. One of the key drivers of POCT adoption in the cystatin C assay market is the increasing prevalence of renal illnesses, which necessitate regular and dependable monitoring. Traditional laboratory tests frequently have lengthier processing periods, which can delay diagnosis and treatment. POCT tackles this issue by providing instantaneous results, enabling healthcare providers to make more timely clinical choices and take necessary interventions. Furthermore, the increased emphasis on personalised medicine and patient-centered treatment has driven up demand for POCT. Personalised treatment regimens necessitate accurate and quick diagnostic information, which POCT may deliver.
Cystatin C assays are typically more expensive than standard creatinine testing. The high cost can be a barrier to widespread adoption, especially in low- and middle-income nations with tight healthcare resources. Healthcare practitioners and patients are unaware of the benefits of cystatin C over creatinine when assessing renal function. Many doctors continue to use creatinine due to familiarity and established practice, which can impede the transition to cystatin C testing. Navigating the complex regulatory environment for diagnostic assays can be difficult. Obtaining regulatory approvals from agencies such as the FDA and EMA takes a significant amount of time and resources, which can cause product launches and market entrance to be delayed.
Insights by Product
The kits segment accounted for the largest market share over the forecast period 2023 to 2033. The rising prevalence of chronic kidney disease (CKD) and acute kidney injury (AKI) worldwide increases demand for diagnostic testing, particularly cystatin C assay kits. Assay technology innovations have resulted in the development of kits that are more sensitive, specific, and user-friendly. These developments increase the accuracy and efficiency of cystatin C measurements. The adoption of POCT is a major driver in the kits segment. POCT necessitates small, dependable, and user-friendly kits that can produce quick results, making cystatin C assay kits excellent for these purposes.
Insights by Sample Type
The blood samples segment is dominating the market with the largest market share over the forecast period 2023 to 2033. Blood samples give a more precise and reliable evaluation of cystatin C levels that is less affected by factors such as muscle mass, making them the recommended method for measuring kidney function. With the increased prevalence of chronic kidney disease (CKD) and acute kidney injury (AKI), there is a greater demand for precise diagnostic tools such as blood-based cystatin C assays. Cystatin C assays performed on blood are crucial for the early detection and monitoring of kidney disorders, allowing for prompt intervention and improved patient outcomes. The introduction of blood-based cystatin C assays is driven by an increased awareness of the necessity of early detection and regular monitoring of kidney function.
Insights by Application
The diagnostics segment is dominating the market with the largest market share over the forecast period 2023 to 2033. The rising prevalence of chronic kidney disease (CKD) and acute kidney injury (AKI) worldwide is increasing the demand for dependable diagnostic tools, such as cystatin C tests, in both clinical and research settings. The growing emphasis on personalised medicine has increased demand for accurate biomarkers such as cystatin C, which can provide useful insights into specific patient profiles, assisting with personalised treatment strategies and improving patient outcomes. Rising global healthcare expenditure, combined with expenditures in research infrastructure, promotes the use of improved diagnostic technologies and collaboration between diagnostic corporations and research institutions.
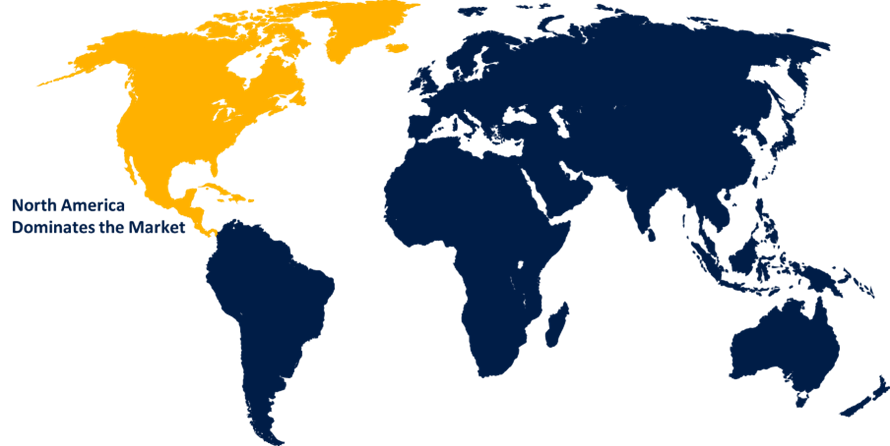
Insights by Region
Get more details on this report -
North America is anticipated to dominate the Cystatin C Assay Market from 2023 to 2033. This region's dominance stems from a mix of modern healthcare infrastructure, high healthcare spending, and rising awareness of chronic kidney disease (CKD) and acute kidney injury (AKI). The availability of well-established healthcare institutions equipped with cutting-edge diagnostic technologies promotes the use of modern tests. Higher healthcare expenditure enables the use of newer and more expensive diagnostic tests, such as cystatin C assays. The move to point-of-care testing is a key trend, driven by the demand for quick and precise diagnosis. POCT devices for cystatin C assays provide convenience and rapid results, facilitating timely clinical decision-making.
Asia Pacific is witnessing the fastest market growth between 2023 to 2033. The cystatin C assay market in Asia-Pacific is expanding rapidly, owing to a combination of factors such as increased healthcare awareness, the prevalence of chronic kidney disease (CKD), and improved healthcare infrastructure. Economic expansion in countries such as China and India is driving more healthcare spending, enabling for the use of more complex and expensive diagnostic procedures. The demographic transition towards an ageing population in many Asia-Pacific countries raises the prevalence of age-related kidney disorders, emphasising the importance of precise kidney function tests. The growing interest in personalised healthcare solutions highlights the need for precise biomarkers such as cystatin C to modify treatment approaches for kidney disease patients.
Recent Market Developments
- In March 2022, Gentian, a key player in the cystatin C test industry, announced that TüV SÜD has certified its Cystatin C and GCAL assays under IVDR (In-Vitro Diagnostic Regulation).
Major players in the market
- Abbott (U.S.)
- Roche Diagnostics Limited (Switzerland)
- Siemens Healthcare GmbH (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Randox Laboratories Ltd. (U.K.)
- DiaSys Diagnostic Systems GmbH (Germany)
- Bio-Techne (U.S.)
- Gentian Diagnostics ASA (Norway)
- Getein Biotech, Inc. (China)
- Agilent Technologies, Inc. (U.S.)
- Abcam plc. (U.K.)
- Sino Biological, Inc. (China)
- Eurolyser Diagnostica GmbH (Austria)
Market Segmentation
This study forecasts revenue at global, regional, and country levels from 2023 to 2033.
Cystatin C Assay Market, Product Analysis
- Kits
- Reagents
- Analyzers
Cystatin C Assay Market, Sample Type Analysis
- Blood
- Urine
Cystatin C Assay Market, Application Analysis
- Diagnostics
- Research
Cystatin C Assay Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?