Global Hernia Mesh Devices Market Size To Worth USD 7.56 Billion By 2033 | CAGR Of 5.42%
Category: HealthcareGlobal Hernia Mesh Devices Market Size to worth USD 7.56 Billion by 2033
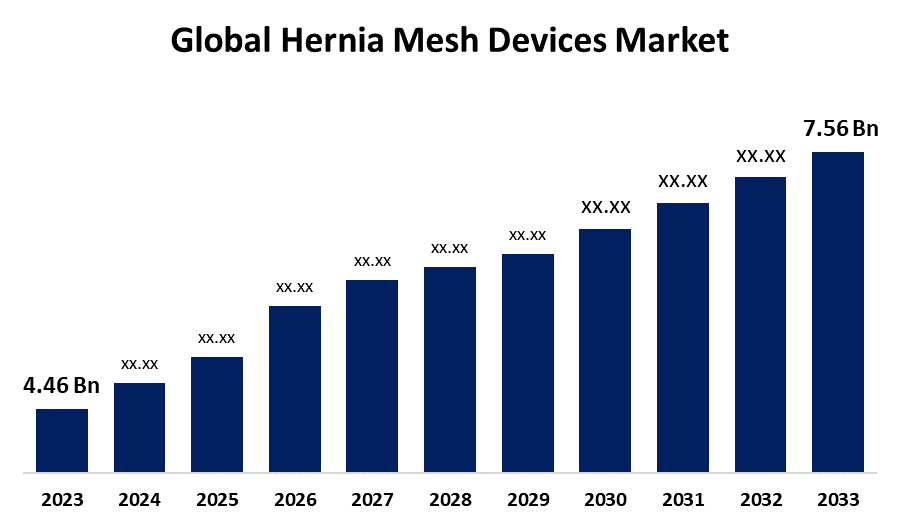
According to a research report published by Spherical Insights & Consulting, the Global Hernia Mesh Devices Market Size is to Grow from USD 4.46 Billion in 2023 to USD 7.56 Billion by 2033, at a Compound Annual Growth Rate (CAGR) of 5.42% during the projected period.
Get more details on this report -
Browse key industry insights spread across 231 pages with 120 Market data tables and figures & charts from the report on the "Global Hernia Mesh Devices Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Synthetic Mesh, Biologic Mesh, and Composite Mesh), By Hernia Type (Inguinal, Incisional, Femoral, Umbilical and Other Hernias), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033." Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/hernia-mesh-devices-market
The injured tissue around hernias can be repaired with the use of a medical device known as hernia mesh. Ligaments, staples, or glue are required to enable tissues to grow into the device, which is composed of both absorbable and non-absorbable materials. During hernia surgery, it is put into the patient's upper stomach, groin, or abdomen to reinforce the hernia repair and reduce the chance of recurrence. Additionally, it improves patient outcomes by reducing the amount of time needed for recovery and surgery. As a result, hernia mesh devices are widely used in clinics, hospitals, and ambulatory surgical centres. The Repairing abdominal wall hernias is one of the procedures that general surgeons perform most frequently. It is used to treat a variety of hernia repairs, including inguinal, incisional, and umbilical hernias. Hernias are a prevalent medical disease that impact millions of individuals worldwide. Because of things like obesity, aging populations, and sedentary lifestyles, hernias are becoming more and more common. This increases the demand for hernia mesh devices and, eventually, hernia repair treatments. The safety and effectiveness of surgical hernia repair operations have been enhanced by technological advancements in surgical techniques, including minimally invasive treatments like this method. However, Financial challenges may make it more difficult to utilize these devices, especially in places where healthcare resources are limited or where payment is a challenge.
The synthetic mesh segment is anticipated to hold the greatest share of the global hernia mesh devices market during the projected timeframe.
Based on the product type, the global hernia mesh devices is divided into synthetic mesh, biologic mesh, and composite mesh. Among these, the synthetic mesh segment is anticipated to hold the greatest share of the global hernia mesh devices market during the projected timeframe. Synthetic meshes dominated the hernia mesh industry due to their wide product availability, low cost, and ease of purchasing product materials. Synthetic meshes led the mesh device industry in terms of sales, largely because of their recent product releases, affordability, and ease of availability. Moreover, synthetic meshes are often more cost-effective for most patients and have fewer surgical complications than biological meshes.
The inguinal hernia segment is anticipated to hold the largest share of the global hernia mesh devices market during the projected timeframe.
Based on the hernia type, the global hernia mesh devices is divided into inguinal, incisional, femoral, Umbilical and other hernias. Among these, the inguinal hernia segment is anticipated to hold the largest share of the global hernia mesh devices market during the projected timeframe. In general surgery, inguinal hernia repair is the most common procedure. Surgeons do inguinal hernia corrections on a daily basis. When they first appear, most people have some kind of groin protrusion or discomfort. To avoid problems, medical specialists suggest treating any symptomatic hernia. Tension-free repair and defect closure may be achieved using an open or laparoscopic procedure.
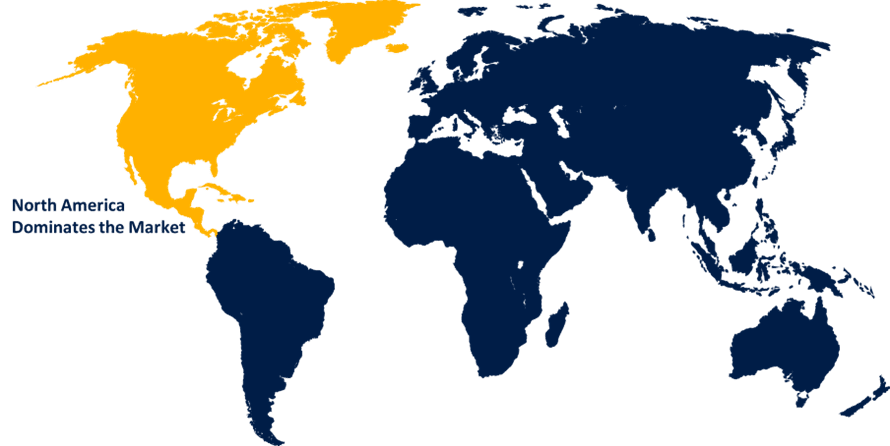
North America is expected to hold the largest share of the global hernia mesh devices over the forecast period.
Get more details on this report -
North America is expected to hold the largest share of the global hernia mesh devices over the forecast period. The key contributing factors include the vast number of persons affected by the illness, improved healthcare facilities, sedentary lifestyles, and the increasing use of surgical meshes as a treatment option. Patients now have easy access to exact compensation codes and ratios because to the efforts of government and industry players. The aging population, increased frequency of hernia recurrence, and lack of activity are some of the factors propelling the market rise in North America. The National Centre for Biotechnology Information states that incisional and ventral hernias were rather prevalent in the US in March 2023. Ventral hernias affect about 20% of people, and up to 30% of midline abdominal injuries result in incisional hernias.
Asia Pacific is predicted to grow at the fastest pace in the global hernia mesh devices during the projected timeframe. Several factors driving this industry include rising healthcare reimbursements, medical tourism, affordable care, and technology advancements. Furthermore, due to a substantial patient population, Asian countries, China and Japan in particular are seeing a sharp increase in demand for hernia repair tools. Owing to the large number of cases that go undiagnosed and untreated, the region is expected to expand significantly, which would propel the market's growth in this field.
Major vendors in the global hernia mesh devices include Johnson & Johnson Services, Inc., C. R. Bard, Inc., W. L. Gore & Associates, Inc., Atrium Medical (Getinge Group), LifeCell, B. Braun SE, Baxter, Cook, Herniamesh S.r.l.,Ethicon, B. Braun Melsungen AG., PRIMEQUAL SA, Becton, Dickinson, and Company, Deep Blue Medical Inc, Others.
Recent Developments
- In April 2022 – The FDA granted Ariste Medical, a medical device firm, permission under section 501(k) to distribute drug-embedded synthetic hernia mesh in the United States.
- In July 2022 - To market its hernia repair solutions, Deep Blue Medical Advances, Inc. increased its venture financing to over USD 7.0 million.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global hernia mesh devices based on the below-mentioned segments:
Global Hernia Mesh Devices Market, By Product Type
- Synthetic mesh
- Biologic mesh
- Composite mesh
Global Hernia Mesh Devices Market, By Hernia Type
- Inguinal
- Umbilical
- Incisional
- Femoral
- Others
Global Hernia Mesh Devices Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?