Global Medical Device Package Validation Market Size To Worth By 2033 | CAGR of 8.59%
Category: HealthcareGlobal Medical Device Package Validation Market Size To Worth By 2033
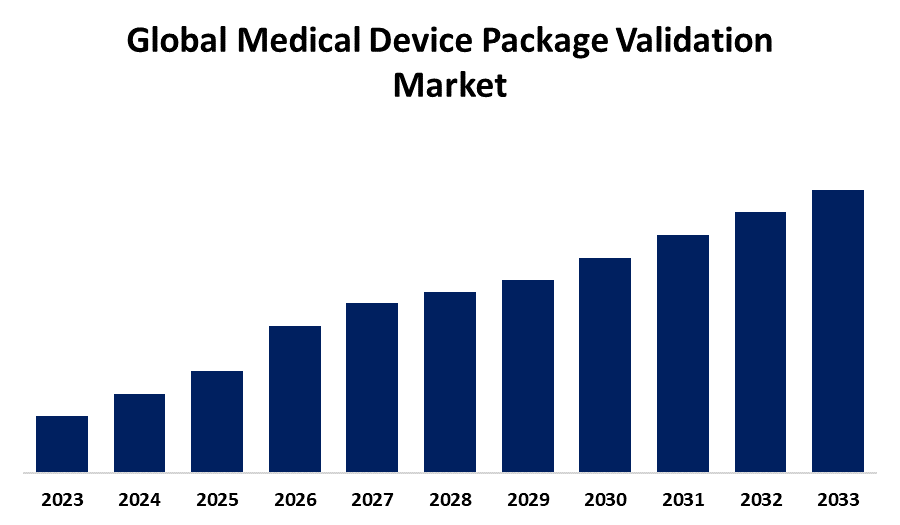
According to a research report published by Spherical Insights & Consulting, the Global Medical Device Package Validation Market Size is Expected to Hold a Significant Share by 2033, at a CAGR of 8.59% during the forecast period 2023-2033.
Get more details on this report -
Browse key industry insights spread across 248 pages with 110 Market data tables and figures & charts from the report on the "Global Medical Device Package Validation Market Size, Share, and COVID-19 Impact Analysis, By Testing Type (Physical Testing, Microbial Testing, Chemical Testing, and Visual Testing), By Device Class (Class I Devices, Class II Devices, and Class III Devices), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033." Get Detailed Report Description Here:https://www.sphericalinsights.com/reports/medical-device-package-validation-market
The identification and control of manufacturing factors and materials that affect a packaged product's ability to meet acceptance standards is the entirety of the packaging validation process. Identifying the optimal windows for each significant variable allows process control and assurance to meet the device package requirements. The market for medical device package validation is driven by stringent standards that ensure the safety, efficacy, and integrity of medical equipment throughout its lifecycle. The regulatory scrutiny forces manufacturers to invest in robust systems that meet international standards, which drives the market for medical device package validation to rise. The primary drivers of the medical device packaging validation market are increased focus on infection prevention and patient safety, stricter regulations requiring robust packaging, and technological developments in medical devices that necessitate specialized packaging to preserve the sterility and integrity of complex healthcare. However, the market for medical device package validation is restricted by several issues, such as changing technology standards, high costs, demanding compliance requirements, and regulatory obstacles.
The physical testing segment is predicted to hold the greatest market share through the forecast period.
Based on the testing type, the medical device package validation market is classified into physical testing, microbial testing, chemical testing, and visual testing. Among these, the physical testing segment is predicted to hold the greatest market share through the forecast period. Strong physical testing techniques are required to prevent package failures that could patient safety and product efficacy. This need is also driven by regulatory regulations.
The class II devices segment is anticipated to hold the greatest market share during the projected timeframe.
Based on the device class, the medical device package validation market is divided into class I devices, class II devices, and class III devices. Among these, the class II devices segment is anticipated to hold the greatest market share during the projected timeframe. Class II devices are complex and varied, necessitating extensive testing methods. Such as specialized validation solutions that meet industry standards and legal requirements are required.
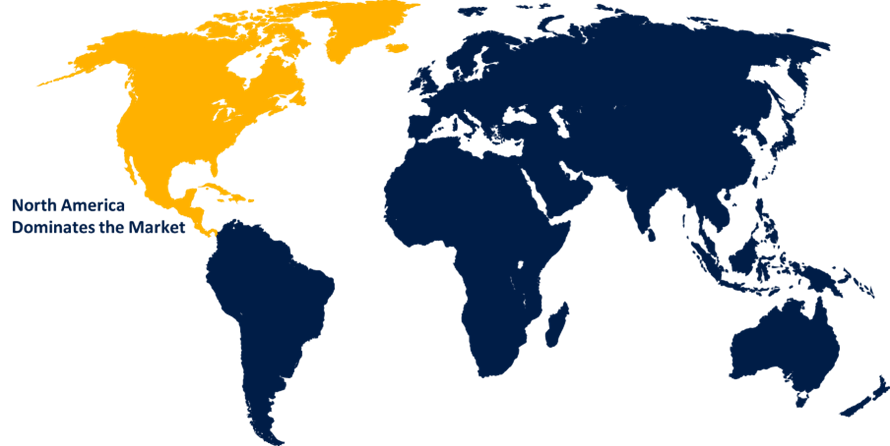
North America is estimated to hold the largest share of the medical device package validation market over the forecast period.
Get more details on this report -
North America is estimated to hold the largest share of the medical device package validation market over the forecast period. Strong healthcare infrastructure, strict regulatory frameworks including FDA regulations, and the concentration of top medical device manufacturers are the reasons for the market supremacy. In order to ensure adherence to strict quality and safety standards, these elements contribute to the region's high demand for comprehensive package validation services.
Asia Pacific is predicted to have the highest CAGR growth in the medical device package validation market over the forecast period. Package validation solutions have grown more quickly in the APAC region as a result of efforts to standardize regulatory requirements throughout APAC nations and the increased focus on quality assurance in the production of medical devices.
Major key players in the medical device package validation market include Glatfelter Corporation, Berry Global Inc., Amcor Plc., Wetspak, Life Science Outsourcing, Inc., Pro-Tech Design & Manufacturing, WuXi AppTec Medical Device Testing, Nelson Labs, Keystone Package Testing, Eurofins Scientific, UL Solutions, SteriPack Contract Manufacturing, DDL, Inc., and Others.
Recent Development
- In January 2024, in order to satisfy the increasing demand from customers in the consumer health, pharmaceutical, and medical sectors on the North American continent, Amcor Plc announced increases to its thermoforming production capacity.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the medical device package validation market based on the below-mentioned segments:
Global Medical Device Package Validation Market, By Testing Type
- Physical Testing
- Microbial Testing
- Chemical Testing
- Visual Testing
Global Medical Device Package Validation Market, By Device Class
- Class I Devices
- Class II Devices
- Class III Devices
Global Medical Device Package Validation Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Follow Us: LinkedIn | Facebook | Twitter
Need help to buy this report?