Global Pharmacovigilance and Drug Safety Software Market Size To Worth USD 382.12 Million by 2033 | CAGR of 6.74%
Category: HealthcareGlobal Pharmacovigilance and Drug Safety Software Market Size To Worth USD 382.12 Million by 2033
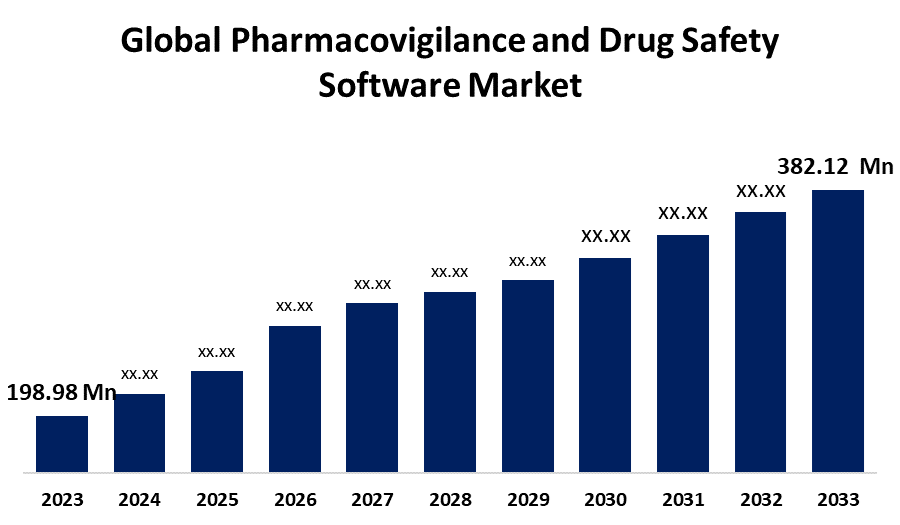
According to a research report published by Spherical Insights & Consulting, the Global Pharmacovigilance and Drug Safety Software Market Size is Expected to Grow from USD 198.98 Million in 2023 to USD 382.12 Million by 2033, at a CAGR of 6.74% during the forecast period 2023-2033.
Get more details on this report -
Browse key industry insights spread across 220 pages with 112 Market data tables and figures & charts from the report on the " Global Pharmacovigilance and Drug Safety Software Market Size, Share, and COVID-19 Impact Analysis, By Functionality (Adverse Event Reporting Software, Signal Detection, and Others), By Delivery Mode (On-Demand and On-Premise), By End-User (Contract Research Organizations (CROs), Pharma and Biotech Companies, Business Process Outsourcing Firms, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033." Detailed Report Description Here: https://www.sphericalinsights.com/reports/pharmacovigilance-and-drug-safety-software-market
The pharmacovigilance and drug safety software market includes digital tools for monitoring, analyzing, and reporting adverse drug reactions and safety-related information in pharmaceuticals and medical products. It is crucial for patient safety and regulatory compliance. The market is expected to grow due to the increasing demand for personalized medicine. The Pharmacovigilance Programme of India in India collects and analyzes adverse drug reactions, ensuring patient safety. The Uppsala Monitoring Centre in Sweden supports pharmacovigilance globally. Major drug safety software used by pharmaceutical companies, regulatory agencies, and healthcare professionals include Oracle Argus Safety, ArisG, ARGUS, and PvNET. The pharmacovigilance and drug safety software market is expanding due to increased adoption by clinical research companies, chronic disease prevalence, strict drug safety regulations, and advanced technology. User-friendly interfaces and continuous software advancements make integration easier. The rise in adverse drug reactions (ADRs) drives demand for robust systems, improving patient safety, optimizing resources, and streamlining reporting processes. However, potential drug withdrawal and inconsistent reporting of adverse drug reactions in middle-income countries may impede market growth.
The adverse event reporting software segment held the greatest share in 2023 and is projected to grow at a significant CAGR during the projected timeframe.
Based on the functionality, the global pharmacovigilance and drug safety software market is categorized as adverse event reporting software, signal detection, and others. Among these, the adverse event reporting software segment held the greatest share in 2023 and is projected to grow at a significant CAGR during the projected timeframe. The segmental expansion is driven by comprehensive data management features, enhancing safety, quality of care, decision-making, transparency, early ADR identification, regulatory compliance, and patient data security.
The on-demand segment accounted for the highest market share of 50.10% in 2023 and is expected to grow at a significant CAGR throughout the projected timeframe.
Based on the delivery mode, the global pharmacovigilance and drug safety software market is categorized as on-demand and on-premise. Among these, the on-demand segment accounted for the highest market share of 50.10% in 2023 and is expected to grow at a significant CAGR throughout the projected timeframe. This is owing to pharmaceutical companies rapidly adopting streamlined accessibility, lower infrastructure costs, and flexible SaaS models, leading to segment growth in real-time data analysis and decision-making.
The pharma and biotech companies segment accounted for the largest market share of 38.67% of the share in 2023 and is predicted to grow at a substantial CAGR during the forecast period.
Based on the end-user, the global pharmacovigilance and drug safety software market is categorized as contract research organizations (CROs), pharma and biotech companies, business process outsourcing firms, and others. Among these, the pharma and biotech companies segment accounted for the largest market share of 38.67% of the share in 2023 and is predicted to grow at a substantial CAGR during the forecast period. The segment's growth is driven by increasing pharmacovigilance software usage, technology advancements, data accuracy, drug discovery complexity, screening services, molecular studies, post-marketing surveillance, R&D activities, and regulatory compliance.
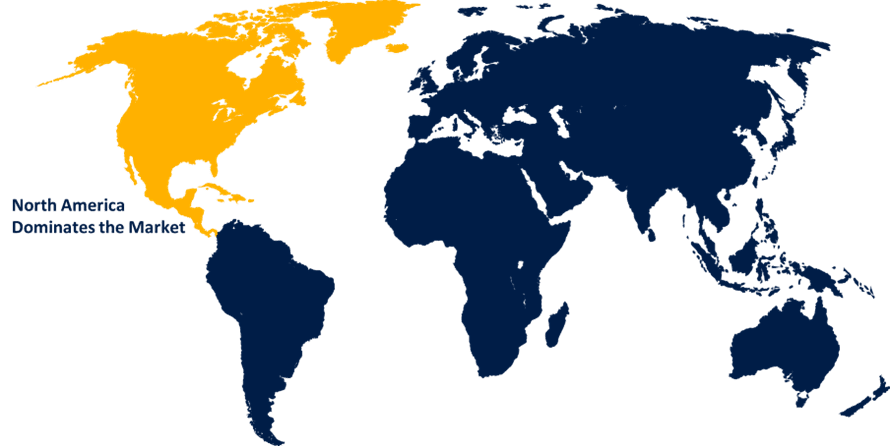
North America is expected to hold the greatest share of the global pharmacovigilance and drug safety software market over the forecast period.
Get more details on this report -
North America is expected to hold the greatest share of the global pharmacovigilance and drug safety software market over the forecast period. Government-aided initiatives like the FDA initiative and Mini-Sentinel project are driving increased usage rates in the drug safety sector, driven by strict regulations, chronic diseases, clinical trials, and outsourcing services.
Asia Pacific is predicted to hold the fastest-growing region of the global pharmacovigilance and drug safety software market throughout the estimated period. Asia Pacific businesses, including Singapore, South Korea, and Taiwan, are renowned for cost-cutting, clinical trials, pharmacovigilance, and drug safety software, due to strict government controls.
Major key players in the global pharmacovigilance and drug safety software market are IQVIA, Genpact, Aris Global, Accenture, IBM, Capgemini, Paraxel International Corporation, Cognizant, United BioSource Corporation, Ennov Solutions Inc., Veeva Systems, and others.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In August 2024, Shri Narendra Modi launched 'Digital India', an indigenously developed ADRMS software for the Pharmacovigilance Programme of India (PvPI), with the Ministers of Health & Family Welfare and Chemicals and Fertilizers. PvPI's ADRMS software is India's first medical product safety database, enabling users to report adverse events related to medicines and medical devices.
- In February 2024, The FDA utilized an AI named Information Visualization Platform (InfoViP) to enhance its pharmacovigilance efforts by reviewing adverse event reports and standardizing Risk Evaluation and Mitigation Strategies data. InfoViP utilizes natural language processing to visualize temporal data, extract clinical concepts from case safety reports, classify reports based on information quality, and enable causality assessments using assessable ICSRs.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the global pharmacovigilance and drug safety software market based on the below-mentioned segments:
Global Pharmacovigilance and Drug Safety Software Market, By Functionality
- Adverse Event Reporting Software
- Signal Detection
- Other Safety Assessment
Global Pharmacovigilance and Drug Safety Software, By Delivery Mode
- On-Demand
- On-Premise
Global Pharmacovigilance and Drug Safety Software Market, By End-User
- Clinical Research Organizations (CROs)
- Pharma and Biotech Companies
- Business Process Outsourcing Firms
- Others
Global Pharmacovigilance and Drug Safety Software Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?