Global Vaccine Contract Manufacturing Market Size To Worth USD 8.88 Billion By 2033 | CAGR Of 11.06%
Category: HealthcareGlobal Vaccine Contract Manufacturing Market Size To Worth USD 8.88 Billion By 2033
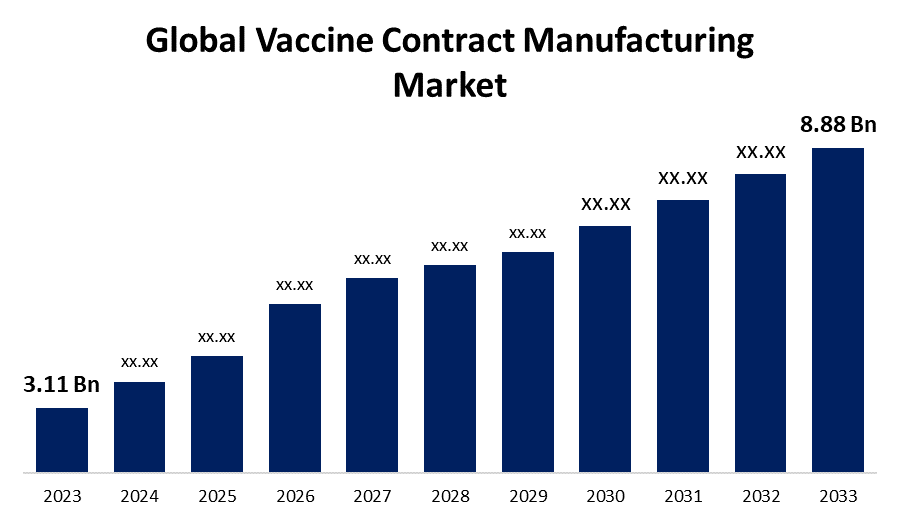
According to a research report published by Spherical Insights & Consulting, The Global Vaccine Contract Manufacturing Market Size is Expected to Grow from USD 3.11 Billion in 2023 to USD 8.88 Billion by 2033, at a CAGR of 11.06% during the forecast period 2023-2033.
Get more details on this report -
Browse key industry insights spread across 240 pages with 110 Market data tables and figures & charts from the report on the "Global Vaccine Contract Manufacturing Market Size, Share, and COVID-19 Impact Analysis, By Type (Attenuated, Inactivated, Subunit-based, Toxoid-based, and DNA-based), By Workflow (Downstream, and Upstream), By Application (Human Use, and Veterinary), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033." Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/vaccine-contract-manufacturing-market
Vaccine is a preparation that is used to stimulate the body’s immune response against diseases. Vaccines are usually administered through needle injections, but some can be administered by mouth or sprayed into the nose. Vaccine contract manufacturing is when a third party is hired to make vaccines for a company or organization. The third party, or contract manufacturing organization (CMO), is responsible for the production, packaging, and labeling of the vaccines. CMOs have the infrastructure, expertise, and facilities to make vaccines. The increasing demand for vaccines serves as a crucial driver propelling the expansion of the vaccine contract manufacturing market. As the global population continues to prioritize preventive healthcare measures, the need for proficient vaccine production escalates. Vaccine Contract Manufacturing offers pharmaceutical companies the flexibility to extent up production swiftly to meet this rising demand. Additionally, outsourcing manufacturing to specialized facilities enables companies to focus on research and development, accelerating vaccine innovation. In the vaccine contract manufacturing market, strict quality control and regulatory acquiescence are significant barriers. To guarantee product safety and efficacy, strict adherence to quality standards, such as Good Manufacturing Practices (GMP), necessitates large expenditures in infrastructure and procedures. Complying with a range of regulatory requirements across different locations increases manufacturing operations' density and expenses. Any departure from these guidelines runs the danger of a product recall or regulatory penalties, which might damage the reliability of the industry and limit future growth projection.
The attenuated segment is predicted to hold the largest market share through the forecast period.
Based on the vaccine type, the vaccine contract manufacturing market is classified into attenuated, inactivated, subunit-based, toxoid-based, and DNA-based. Among these, the attenuated segment is predicted to hold the largest market share through the forecast period. This due to several factors, Attenuated vaccines, which contain weaken forms of pathogens, require specialized expertise and facilities for safe and effective production. Contract manufacturing organizations (CMOs) with experience in handling live, attenuated strains are in high demand. Moreover, attenuated vaccines often have a long history of efficacy, making them essential for preventing various diseases.
The downstream segment is anticipated to hold the highest market share during the projected timeframe.
Based on the application, the vaccine contract manufacturing market is divided into downstream, and upstream. Among these, the downstream segment is anticipated to hold the highest market share during the projected timeframe. This is because it encompasses significant processes like formulation, fill-and-finish, packaging, and distribution. These stages are critical for ensuring vaccine safety, efficacy, and accessibility. Vaccine developers often rely on contract manufacturing organizations (CMOs) to handle these complex downstream tasks, as they require dedicated equipment, expertise, and regulatory compliance. By outsourcing these crucial steps to CMOs, vaccine developers can streamline their operations, reduce costs, and expedite vaccine production, making the downstream segment a crucial and leading constituent of the vaccine contract manufacturing market.
The human use segment dominates the market with the largest market share through the forecast period.
Based on the application, the vaccine contract manufacturing market is categorized into human use, and veterinary. Among these, the human use segment dominates the market with the largest market share through the forecast period. This is due to the important and constant demand for human vaccines. Vaccination is a keystone of public health programs globally, addressing various infectious diseases and preventing their spread. As a result, pharmaceutical companies and governments regularly require contract manufacturing services to meet the production needs of human vaccines. This constant demand, along with the need for dedicated expertise in human vaccine manufacturing, drives the dominance of the human-use segment within the vaccine contract manufacturing market.
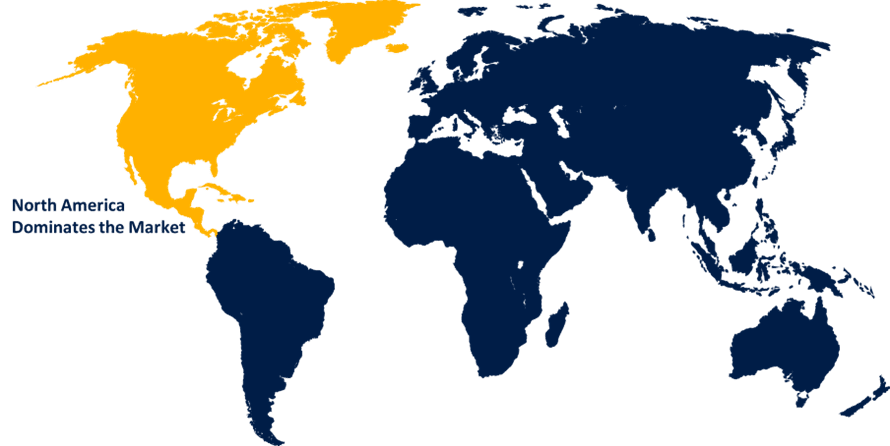
North America is estimated to hold the largest share of the vaccine contract manufacturing market over the forecast period.
Get more details on this report -
North America is estimated to hold the largest share of the vaccine contract manufacturing market over the forecast period. North America boasts a well-developed pharmaceutical industry with numerous established pharmaceutical and biotech companies that specialize in vaccine research, development, and manufacturing, driving growth of the region. The U.S. boasts a robust pharmaceutical industry with numerous leading pharmaceutical and biotech companies that specialize in vaccine research, development, and manufacturing. Furthermore, FDA's rigorous oversight and expertise in vaccine regulation provide assurance to global markets, elevating the desirability of vaccines manufactured in the U.S. The region has one of the highest healthcare expenditures globally, reflecting significant investments in healthcare infrastructure, research, and development.
Asia Pacific is expected to grow the fastest during the forecast period. Primarily, it boasts a large and growing population, leading to increased demand for vaccines. Asia-Pacific has a robust pharmaceutical and biotechnology industry, attracting global vaccine developers who seek cost-effective manufacturing solutions. Moreover, the region benefits from a skilled workforce and advanced manufacturing capabilities. Furthermore, favorable regulatory environments and lower labour costs make it an attractive destination for vaccine contract manufacturing. As a result, Asia-Pacific continues to be a major contributor to the global vaccine contract manufacturing market growth.
Major key players in the vaccine contract manufacturing market include Fujifilm Diosynth Biotechnologies, AGC Biologics, Catalent, Inc., Charles River Laboratories, Emergent BioSolutions, Samsung Biologics, IDT Biologika GmbH, KBI Biopharma, Lonza Group, WuXi Biologics, Merck KGaA, Recipharm AB, Richter-Helm BioLogics GmbH & Co. KG, Seqirus (CSL Limited), and Others.
Recent Developments
In June 2024, South Korean biotech firm SK Bioscience is to acquire the German CDMO-specializing company IDT Biologika at 339 billion won ($244 million) to further sharpen and diversify its pipeline for global markets.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the vaccine contract manufacturing market based on the below-mentioned segments:
Global Vaccine Contract Manufacturing Market, By Vaccine Type
- Attenuated
- Inactivated
- Subunit-based
- Toxoid-based
- DNA-based
Global Vaccine Contract Manufacturing Market, By Workflow
- Downstream
- Upstream
Global Vaccine Contract Manufacturing Market, By Application
- Human Use
- Veterinary
Global Vaccine Contract Manufacturing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Follow Us: LinkedIn | Facebook | Twitter
Need help to buy this report?