Asia Pacific Laboratory Developed Tests Market Size, Share, and COVID-19 Impact Analysis, By Technology (Immunoassays, Molecular Diagnostics, and Flow Cytometry), By Application (Oncology, Genetic Disorders/Inherited Disease, Infectious & Parasitic Diseases, Immunology, and Tablets), and Asia Pacific Laboratory Developed Tests Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareAsia Pacific Laboratory Developed Tests Market Insights Forecasts to 2033
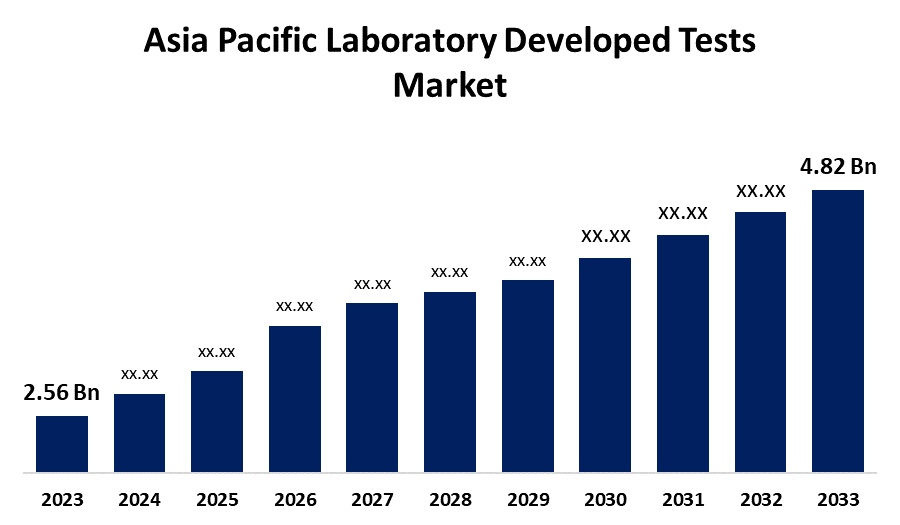
- The Asia Pacific Laboratory Developed Tests Market Size was valued at USD 2.56 Billion in 2023.
- The Market Size is Growing at a CAGR of 6.53% from 2023 to 2033
- The Asia Pacific Laboratory Developed Tests Market Size is Expected to Reach USD 4.82 Billion by 2033
Get more details on this report -
The Asia Pacific Laboratory Developed Tests Market is Anticipated to Exceed USD 4.82 Billion by 2033, growing at a CAGR of 6.53% from 2023 to 2033.
Market Overview
The Asia Pacific laboratory developed tests (LDTs) market refers to the sector encompassing diagnostic tests that are designed, manufactured, and utilized within individual laboratories to meet specific clinical requirements. Unlike commercial in vitro diagnostic (IVD) tests, LDTs are developed in-house and tailored to address unique medical conditions, emerging diseases, and specialized testing needs. These tests play a crucial role in precision medicine, oncology diagnostics, genetic testing, and infectious disease detection, driving advancements in personalized healthcare. The growth of the Asia Pacific LDTs market is primarily driven by the rising prevalence of chronic and infectious diseases, increasing demand for personalized medicine, and advancements in molecular diagnostics. The expansion of healthcare infrastructure and the growing adoption of next-generation sequencing (NGS) technologies further contribute to market development. Additionally, the need for rapid, cost-effective, and highly specialized diagnostic solutions continues to support the increasing utilization of LDTs across the region. Several government initiatives are shaping the regulatory landscape for LDTs in the Asia Pacific region. Authorities in countries such as China, Japan, and India are implementing policies to enhance quality control, standardization, and validation of laboratory-developed tests. Efforts to streamline regulatory frameworks and promote innovation in diagnostic testing are expected to strengthen the market’s growth potential while ensuring patient safety and clinical reliability.
Report Coverage
This research report categorizes the market for the Asia Pacific laboratory developed tests market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Asia Pacific laboratory developed tests market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Asia Pacific laboratory developed tests market.
Asia Pacific Laboratory Developed Tests Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 2.56 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 6.53% |
| 2033 Value Projection: | USD 4.82 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Technology, By Application |
| Companies covered:: | Quest Diagnostics Incorporated, Abbott, Guardant Health, NeoGenomics Laboratories, Siemens Healthcare Private Limited, QIAGEN, Illumina, Inc., F. Hoffmann-La Roche Ltd, and Others. |
| Pitfalls & Challenges: | COVID-19 impact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Asia Pacific laboratory developed tests (LDTs) market is driven by several key factors, including the rising prevalence of chronic diseases such as cancer, cardiovascular disorders, and infectious diseases. The growing demand for personalized medicine and targeted therapies has further accelerated the adoption of LDTs. Advancements in molecular diagnostics, next-generation sequencing (NGS), and biomarker discovery enhance test accuracy and efficiency. Increasing investments in healthcare infrastructure and research and development (R&D) initiatives also contribute to market expansion. Additionally, the need for cost-effective, rapid, and specialized diagnostic solutions fuels the demand for LDTs across clinical and research laboratories in the region.
Restraining Factors
The Asia Pacific laboratory developed tests (LDTs) market faces challenges such as regulatory uncertainties, lack of standardized guidelines, and concerns over test accuracy and reproducibility. High development costs, limited reimbursement policies, and the need for specialized expertise further restrict market growth. Additionally, stringent approval processes hinder widespread adoption of LDTs.
Market Segmentation
The Asia Pacific laboratory developed tests market share is classified into technology and dosage form.
- The molecular diagnostics segment is expected to hold the largest market share through the forecast period.
The Asia Pacific laboratory developed tests market is segmented by technology into immunoassays, molecular diagnostics, and flow cytometry. Among these, the molecular diagnostics segment is expected to hold the largest market share through the forecast period. The prominence of molecular diagnostics can be attributed to its advanced capabilities in detecting and analyzing genetic material, which is crucial for identifying various diseases, including infectious diseases, genetic disorders, and cancers. The increasing demand for personalized medicine and targeted therapies further bolsters the adoption of molecular diagnostic techniques in laboratory-developed tests across the Asia Pacific region.
- The oncology segment dominates the market with the largest market share over the predicted period.
The Asia Pacific laboratory developed tests market is segmented by application into oncology, genetic disorders/inherited disease, infectious & parasitic diseases, immunology, and tablets. Among these, the oncology segment dominates the market with the largest market share over the predicted period. This prominence is attributed to the increasing incidence of cancer in the region, which necessitates advanced diagnostic solutions for early detection and personalized treatment strategies. The rising adoption of molecular diagnostics and next-generation sequencing technologies further enhances the development and utilization of LDTs in oncology applications.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Asia Pacific laboratory developed tests market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Quest Diagnostics Incorporated
- Abbott
- Guardant Health
- NeoGenomics Laboratories
- Siemens Healthcare Private Limited
- QIAGEN
- Illumina, Inc.
- F. Hoffmann-La Roche Ltd
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-added resellers (VARs)
Market Segment
This study forecasts revenue at Asia Pacific, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Asia Pacific laboratory developed tests market based on the below-mentioned segments:
Asia Pacific Laboratory Developed Tests Market, By Technology
- Immunoassays
- Molecular Diagnostics
- Flow Cytometry
Asia Pacific Laboratory Developed Tests Market, By Application
- Oncology
- Genetic Disorders/Inherited Disease
- Infectious & Parasitic Diseases
- Immunology
- Tablets
Need help to buy this report?