Australia Reprocessed Medical Devices Market Size, Share, and COVID-19 Impact Analysis, By Product (Cardiovascular, Laparoscopic, Gastroenterology, General Surgery Devices, Orthopedic Devices, Others), By End User (Hospitals, Diagnostic Centers, Ambulatory Surgical Center), and Australia Reprocessed Medical Devices Market Insights Forecasts to 2032
Industry: HealthcareAustralia Reprocessed Medical Devices Market Insights Forecasts to 2032
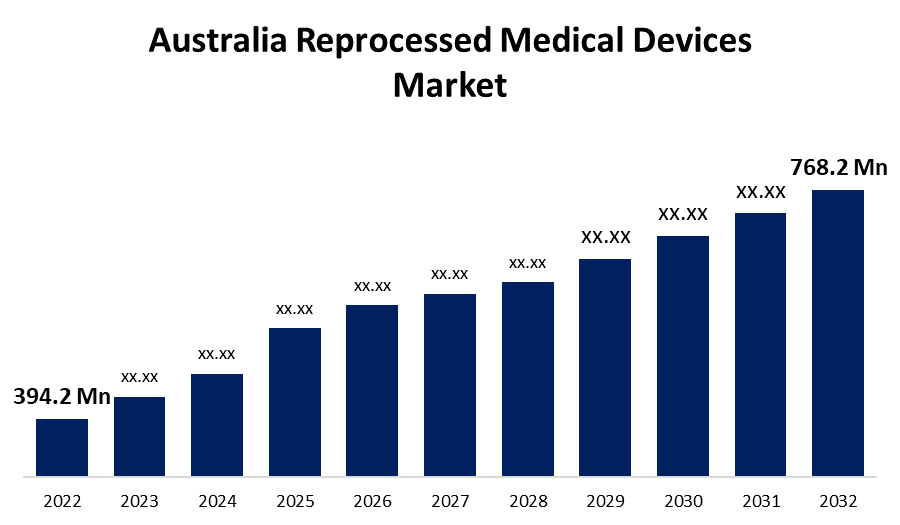
- The Australia Reprocessed Medical Devices Market Size was valued at USD 394.2 Million in 2022.
- The Market Size is Growing at a CAGR of 6.9% from 2022 to 2032.
- The Australia Reprocessed Medical Devices Market Size is Expected to Reach USD 768.2 Million by 2032.
Get more details on this report -
The Australia Reprocessed Medical Devices Market Size is Expected to Reach USD 768.2 Million by 2032, at a CAGR of 6.9% during the forecast period 2022 to 2032.
Market Overview
Reprocessed medical devices are products that have been reprocessed and reused. Cleaning, disinfection, and sterilization are all steps in the reprocessing of a single used device or reusable device. The reprocessed medical devices contain such as gastroenterology devices, cardiology devices, laparoscopy devices, orthopedic/arthroscopic devices, ENT devices, and others such as pulse oximetry sensors and ultrasound catheters. Increased hospital adoption of commercial medical device reprocessing, increased emphasis on reducing medical waste, and the implementation of quality management systems are expected to drive market growth.
Report Coverage
This research report categorizes the market for the Australia reprocessed medical devices market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Australia reprocessed medical devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Australia reprocessed medical devices market.
Australia Reprocessed Medical Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 394.2 Million |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 6.9% |
| 2032 Value Projection: | USD 768.2 Million |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product, By End User, and COVID-19 Impact Analysis. |
| Companies covered:: | Stryker, Medline Industries, LP, Hygia Healthcare, Cleanpart Gmb, ReNu Medical, Inc., SureTek Medical, NEScientific, Inc. and Other Key Vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Reprocessing of medical devices and equipment has received a lot of attention in recent years, not only in healthcare facilities for saving millions of dollars per year, but also among manufacturers who see it as a way to gain a significant competitive advantage. Medical device reprocessing has become one of the most widely used supply chain cost-cutting strategies among hospitals, surgical centers, and other healthcare facilities. This frees up funds for end users to hire more staff, upgrade technology, and improve healthcare quality, among other things. Increased product launch activities to develop newer reprocessing devices or their accessories may drive the growth of the Australia reprocessed medical devices market. Growing partnerships, mergers, and collaborations are major factors driving the high demand for Australia reprocessed medical devices. The joint reprocessing initiative aims to improve the performance of medical device reprocessing programs, which frequently do not provide the hospital with optimal savings results.
Restraining Factors
One of the most serious issues confronting the remanufactured medical device industry is the ongoing concern about safety. To ensure patient safety, reprocessed equipment must be thoroughly cleaned, disinfected, and sterilized to eliminate any pathogens. Infections and other negative outcomes may result from insufficient cleaning and sterilization methods. This problem necessitates strict regulatory monitoring and precise quality control techniques in order to maintain patient trust and confidence in the safety of reprocessed devices.
Market Segment
- In 2022, the cardiovascular segment is expected to hold the largest share of the Australia reprocessed medical devices market during the forecast period.
Based on the product, the Australia reprocessed medical devices market is classified into cardiovascular, laparoscopic, gastroenterology, general surgery devices, orthopedic devices, and others. Among these, the cardiovascular segment is expected to hold the largest share of the Australia reprocessed medical devices market during the forecast period. This can be attributed to the widespread use of these products in cardiovascular surgery and diagnostics. Blood pressure cuffs, cardiac positioning and stabilization devices, blood pressure monitoring, electrophysiology cables, and diagnostic electrophysiology catheters are examples of these devices. The growing number of FDA approvals for reprocessed cardiovascular products is contributing to the segment's size. The increase in cardiovascular surgeries and treatments is driving a demand for more cost-effective alternatives, which is driving segment growth.
- In 2022, the hospitals segment accounted for the largest revenue share over the forecast period.
Based on the end user, the Australia reprocessed medical devices market is segmented into hospitals, diagnostic centers, ambulatory surgical center. Among these, the hospitals segment has the largest revenue share over the forecast period. This can be attributed to the high demand for and use of reprocessed medical devices to reduce the cost and medical waste generation associated with original medical equipment. Aside from the environmental implications, healthcare leaders place a premium on the quality of reprocessed devices; studies have shown that re-used medical instruments can be more reliable than new medical devices.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Australia reprocessed medical devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Stryker
- Medline Industries
- LP
- Hygia Healthcare
- Cleanpart Gmb
- ReNu Medical, Inc.
- SureTek Medical
- NEScientific, Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2022, Medline ReNewal, a company that works with and manages reprocessing programs for over 2,000 healthcare organizations, has opened a new distribution center in Mississippi, United States. The facility serves the region's major hospitals, nursing homes, and military bases. To meet the product needs of its healthcare customers, the company invested in expanding its storage and distribution capacity in Mississippi. The Southaven facility is expected to handle over US$ 350.0 Mn in annual orders.
Market Segment
This study forecasts revenue at regional, and country levels from 2021 to 2032. Spherical Insights has segmented the Australia reprocessed medical devices market based on the below-mentioned segments:
Australia Reprocessed Medical Devices Market, By Product
- Cardiovascular
- Laparoscopic
- Gastroenterology
- General Surgery Devices
- Orthopedic Devices
- Others
Australia Reprocessed Medical Devices Market, By End User
- Hospitals
- Diagnostic Centers
- Ambulatory Surgical Center
Need help to buy this report?