Global Bioprocess Validation Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Extractables and Leachable Testing, Integrity Testing, Bioprocess Residuals Testing, Viral Clearance Testing, Systems Testing, Compatibility Testing, Microbiological Testing, Physiochemical Testing, Bacterial Retention Testing, and Others), By Process Component (Filter Elements, Media Containers And Bags, Bioreactors, Freezing And Thawing Process Bags, Mixing Systems, Tubing, Connectors, Samplers, And Others), By End User (Pharmaceutical Companies, Biotechnology Companies, Contract Development & Manufacturing Organizations, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Bioprocess Validation Market Insights Forecasts to 2033
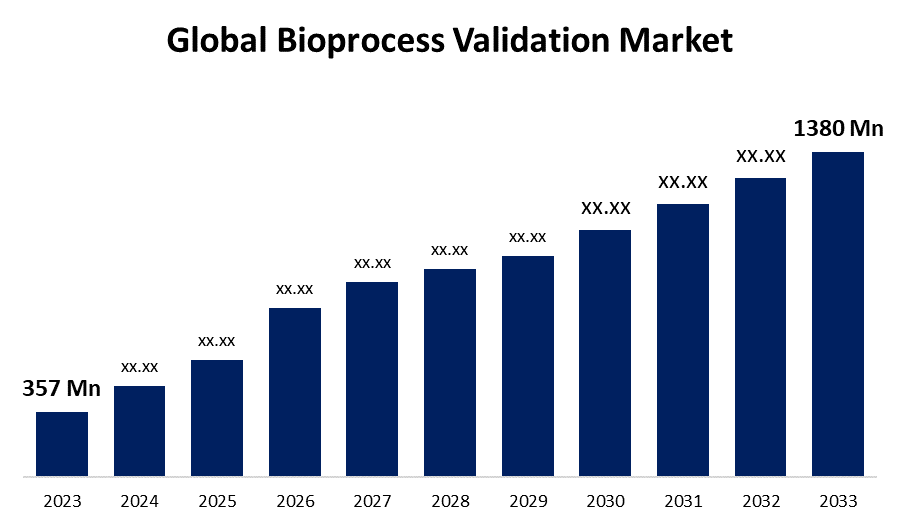
- The Global Bioprocess Validation Market Size was Valued at USD 357 Million in 2023
- The Market Size is Growing at a CAGR of 14.48% from 2023 to 2033
- The Worldwide Bioprocess Validation Market Size is Expected to Reach USD 1380 Million by 2033
- North America is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Bioprocess Validation Market Size is Anticipated to Exceed USD 1380 Million by 2033, Growing at a CAGR of 14.48% from 2023 to 2033.
Market Overview
The procedure of documenting every step, action, and piece of evidence used in the creation of biological and biopharmaceutical products is known as bioprocess validation. The documentation is finished in compliance with current product manufacturing process regulations and US FDA requirements. It ensures adherence throughout the entire process of product testing. Evaluation of the active pharmaceutical ingredients and certain contaminants, such as bacteria, mycoplasma, and endotoxins, is required for bioprocess validation. To be more precise, information is first gathered, evaluated, and then recorded from every phase of a certain project at all critical points. In this way, bioprocess validation provides tangible proof supporting its consistent production of high-quality products. As a result, effective process validation plays a major role in raising the medication's effectiveness, quality, and safety. Government agencies demand this bioprocess validation, and market players adhere to it to produce high-quality products at lower costs. Multiple parameters are examined at each level of the multi-stage bioprocess validation process. To optimize company assets, bioprocess validation makes sure that consistently high-quality products are produced. Pharmaceutical bioprocess validation is an integrated process since the pharmaceutical sector must adhere to the requirements established by many regulatory agencies.
Report Coverage
This research report categorizes the market for the global bioprocess validation market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global bioprocess validation market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global bioprocess validation market.
Global Bioprocess Validation Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 357 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 14.48% |
| 2033 Value Projection: | USD 1380 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Test Type, By Process Component, By End User, By End User |
| Companies covered:: | Eurofins Scientific, Inc., Thermo Fisher Scientific Inc., Biozeen, Pall Corporation, Danaher Corporation, Sartorius Stedium Biotech, Porvair Plc, Labcorp Corporation, Cobetter Filtration equipment Co., Ltd., Corning Inc., Toxikon Corporation, Charles River Laboratories, Cytovance, Lonz Group, and Others |
| Pitfalls & Challenges: | Covid 19 Impact Challanges, Future, Growth and Analysis |
Get more details on this report -
Driving Factors
The global bioprocess validation market underscores the growing need for bioprocess validation, a process that guarantees the security, potential, and effectiveness of biopharmaceutical products and procedures. There is a huge demand for bioprocess validation market services to be outsourced, as well as legal requirements in the healthcare sector to maintain product manufacturing practice compliance. The increasing use of biopharmaceuticals, strict regulations, and the demand for trustworthy validation techniques to satisfy quality standards are the main factors driving this rise. The market is expected to be crucial in preserving the integrity of bioprocesses throughout the world as it develops further.
Restraining Factors
Bioprocess validation is not error-free there are flaws in the testing procedures and validation process phases. Errors arise from internal sources or individuals carrying out the validation procedure. False positives or negatives may appear in the samples, which could change the outcome. These minor mistakes can occasionally result in large losses for the business and possibly restrict market expansion.
Market Segmentation
The global bioprocess validation market share is classified into test type, process component, and end-user.
- The extractable and leachable testing segment dominates the market with the largest market share through the forecast period.
Based on the test type, the global bioprocess validation market is categorized into extractable and leachable testing, integrity testing, bioprocess residuals testing, viral clearance testing, systems testing, compatibility testing, microbiological testing, physiochemical testing, bacterial residuals testing, and others. Among these, the extractable and leachable testing segment dominates the market with the largest market share through the forecast period. Extractable and leachable testing is to identify potentially hazardous substances that may get delivered to the patient together with the medication or drug, extractable and leachable testing is carried out. The focus of the extractable and leachable testing investigations is to ascertain whether hazardous organic and inorganic contaminants impacted the final product's efficacy or safety.
- The filter elements segment is anticipated to grow at the fastest CAGR growth through the forecast period.
Based on the process component, the global bioprocess validation market is categorized into filter elements, media containers and bags, bioreactors, freezing and thawing process bags, mixing systems, tubing, connectors, samplers and others. Among these, the filter elements segment is anticipated to grow at the fastest CAGR growth through the forecast period. The filter elements providing the integrity of the products is an important step in the bioprocess validation process, the filter elements act as the foundation. To produce high-quality products, they eliminate any undesirable particles, including microbes.
- The pharmaceutical companies segment accounted for the largest revenue share through the forecast period.
Based on the end-user, the global bioprocess validation market is categorized into pharmaceutical companies, biotechnology companies, contract development & manufacturing organizations, and others. Among these, the pharmaceutical companies segment accounted for the largest revenue share through the forecast period. Pharmaceutical companies perform research, development, produce, and distribute medications, vaccines, and diagnostic tests to patients to treat, prevent, or test diseases and conditions. Pharmaceutical companies are mainly due to the rise in biopharmaceutical manufacturing and the associated rise in contaminants that need to be tested for, as well as the strict laws and guidelines governing the reliability and caliber of the bioprocesses used in their development.
Regional Segment Analysis of the Global Bioprocess Validation Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
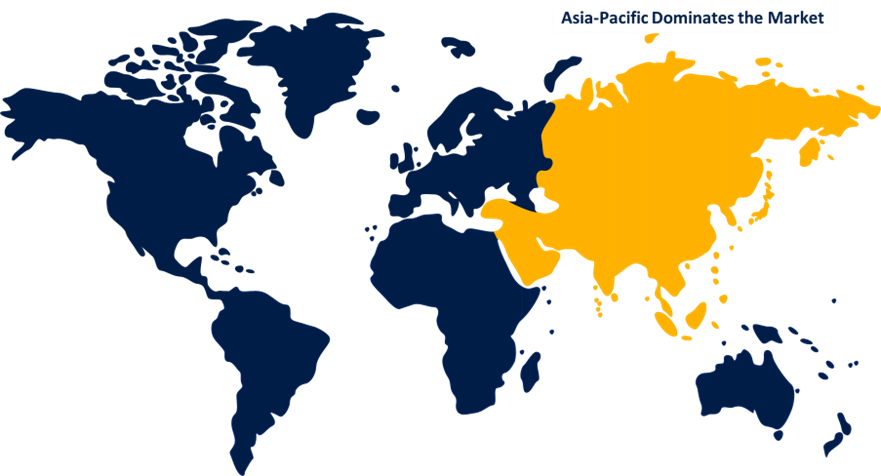
Asia Pacific is anticipated to hold the largest share of the global bioprocess validation market over the predicted timeframe.
Get more details on this report -
Asia Pacific is anticipated to hold the largest share of the global bioprocess validation market over the predicted timeframe. This is due to government-established R&D funds, increased healthcare spending, and technological advancements. Government activities for the growth and development of the biopharmaceutical industry in the Asia Pacific are also supported by a growing awareness of the advantages and benefits of vaccines and biopharmaceutical drugs for the treatment of chronic diseases. The market expansion is anticipated to be driven by growing life science research focused on biologics, the expanding demand for outsourcing bioprocess validation, the expanding biopharmaceutical manufacturing capabilities in Asian nations, and the growing investments made by pharmaceutical and biotechnology businesses. In addition, there are an increasing number of CROs and CDMOs, expanding public awareness of the benefits of biopharmaceutical medications, and positive government measures in numerous Asia Pacific nations to support the expansion of the biotechnology and pharmaceutical industries.
North America is expected to grow at the fastest CAGR of the global bioprocess validation market during the forecast period. This is because of accommodation to important outsourcing services. This causes the production of biologics to increase and life science research to expand, driving the global market. The development of the regional market of North America is further aided by the region's well-established healthcare industry and large pharmacy retail networks, such as pharmacies and consumer value stores.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global bioprocess validation market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Eurofins Scientific, Inc.
- Thermo Fisher Scientific Inc.
- Biozeen
- Pall Corporation
- Danaher Corporation
- Sartorius Stedium Biotech
- Porvair Plc
- Labcorp Corporation
- Cobetter Filtration equipment Co., Ltd.
- Corning Inc.
- Toxikon Corporation
- Charles River Laboratories
- Cytovance
- Lonz Group
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In Jan 2023, Cell Metric X, a high-contrast imager that allows automatic same-day identification of clonally generated cells; Advanced Instruments released it, a global producer of scientific and analytical instruments.
- In July 2022, Vaccizone declared collaboration with Exothera to exploit the latter's Scale-X technology to develop & manufacture the former's COVID vaccine with the bioprocess validation.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global bioprocess validation market based on the below-mentioned segments:
Global Bioprocess Validation Market, By Test Type
- Extractables and Leachables Testing
- Integrity Testing
- Bioprocess Residuals Testing
- Viral Clearance Testing
- Systems Testing
- Compatibility Testing
- Microbiological Testing
- Physiochemical Testing
- Bacterial Retention Testing
- Adsorption Testing
- Others
Global Bioprocess Validation Market, By Process Component
- Filter elements
- Media containers and bags
- Bioreactors
- Freezing and thawing process bags
- Mixing systems
- Tubing
- Connectors
- Samplers
- Others
Global Bioprocess Validation Market, By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Development & Manufacturing Organizations
- Others
Global Bioprocess Validation Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Need help to buy this report?