Canada Clot Management Devices Market Size, Share, and COVID-19 Impact Analysis, By Product (Neurovascular Embolectomy Devices, Embolectomy Balloon Catheters, Percutaneous Thrombectomy Devices, Catheter-Directed Thrombectomy (CDT) devices, and Inferior vena cava filters (IVCF)), By End-use (Diagnostic centers and Hospitals), and Canada Clot Management Devices Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareCanada Clot Management Devices Market Insights Forecasts to 2033
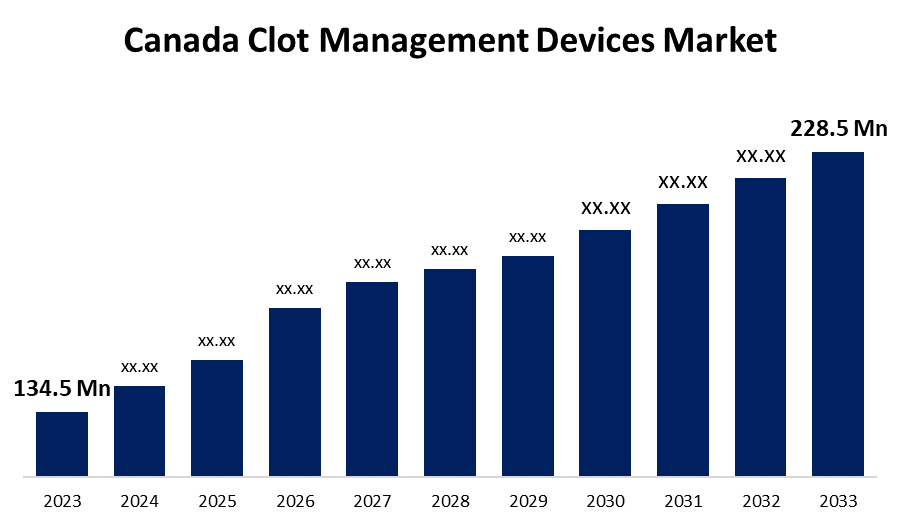
- The Canada Clot Management Devices Market Size was valued at USD 134.5 Million in 2023.
- The Market Size is Growing at a CAGR of 5.44% from 2023 to 2033
- The Canada Clot Management Devices Market Size is expected to reach USD 228.5 Million by 2033
Get more details on this report -
The Canada Clot Management Devices Market Size is anticipated to exceed USD 228.5 Million by 2033, Growing at a CAGR of 5.44% from 2023 to 2033. The increasing need for effective thrombectomy/embolectomy devices, awareness about new approaches to clot management, and technological improvement are driving the growth of the clot management devices market in the Canada.
Market Overview
Clot management devices are medical devices that are used for managing blood coagulums to restore proper blood circulation. These devices are used to treat potentially fatal complications including pulmonary embolism (PE) and deep vein thrombosis (DVT) as well as a regular intervention during percutaneous coronary procedures. Catheter-directed thrombolysis (CDT) catheters, thrombectomy devices, embolectomy balloon catheters, and inferior venous cava filters (IVCF) are some of the devices used for clot management. The high prevalence of atherosclerotic risk factors which is associated with cardiovascular and peripheral vascular systems surges the demand for clot management. Further, there are continuous technological advancements and innovations for safer and more efficient therapies while lessening the burden of health problems associated with vascular disease. For instance, thrombectomy devices are miniaturized devices and comprised of real-time imaging and navigation systems for precise guidance and optimizing clot removal.
Report Coverage
This research report categorizes the market for the Canada clot management devices market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Canada clot management devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Canada clot management devices market.
Canada Clot Management Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 134.5 Million |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 5.44% |
| 2033 Value Projection: | USD 228.5 Million |
| Historical Data for: | 2019 - 2022 |
| No. of Pages: | 178 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By End-use. |
| Companies covered:: | Boston Scientific Corp, Medtronic Plc, Terumo Corp, Teleflex Inc, Penumbra Inc, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Ischaemic stroke is the most common type of stroke in Canada having more than 50,000 cases and 14,000 deaths from stroke every year. The increasing prevalence of ischaemic stroke surges the need for effective thrombectomy/embolectomy devices for improving functional independence and clinical outcomes are driving the market. Furthermore, technological advancements and innovative devices including catheters, filters, and stents for providing better coagulum retrieval and dissolution capabilities are propelling the market growth.
Restraining Factors
The unavailability of skilled professionals and lack of healthcare infrastructure in the country are challenging the Canada clot management devices market.
Market Segmentation
The Canada Clot Management Devices Market share is classified into product and end-use.
- The percutaneous thrombectomy devices segment dominated the market with the largest market share in 2023.
The Canada clot management devices market is segmented by product into neurovascular embolectomy devices, embolectomy balloon catheters, percutaneous thrombectomy devices, catheter-directed thrombectomy (CDT) devices, and inferior vena cava filters (IVCF). Among these, the percutaneous thrombectomy devices segment dominated the market with the largest market share in 2023. Percutaneous thrombectomy devices are effectively used for clot removal by inserting a long, thin, hollow tube called a catheter into the site of the embolism using X-ray guidance. The distinctive advantages of this device over traditional invasive procedures for treating conditions like Deep Vein Thrombosis (DVT) and arterial thrombosis are driving the market.
- The hospitals segment accounted for the largest market share during the forecast period.
The Canada clot management devices market is segmented by end-use into diagnostic centers and hospitals. Among these, the hospitals segment accounted for the largest market share during the forecast period. Clot management devices are used in hospitals for conditions like DVT. The ideal setting provided by hospitals including specialized departments, advanced labs, and operating rooms in hospitals for clot management procedures are driving the market growth. The telestroke services provided by hospitals to save time during stroke treatment are contributing to drive market growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Canada clot management devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Boston Scientific Corp
- Medtronic Plc
- Terumo Corp
- Teleflex Inc
- Penumbra Inc
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In August 2022, The Health Canada-approved Vena BDAC combines the balloon guide catheters and distal access catheters that are currently used in thrombectomy to remove clots from the brains of stroke patients.
Market Segment
This study forecasts revenue at Canada, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Canada Clot Management Devices Market based on the below-mentioned segments:
Canada Clot Management Devices Market, By Product
- Neurovascular Embolectomy Devices
- Embolectomy Balloon Catheters
- Percutaneous Thrombectomy Devices
- Catheter-Directed Thrombectomy (CDT) devices
- Inferior vena cava filters (IVCF)
Canada Clot Management Devices Market, By End-use
- Diagnostic centers
- Hospitals
Need help to buy this report?