Global Cardiovascular Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Study Design (Interventional, Observational, and Expanded Access), By Indication (Coronary Artery Disease, Cardiac Arrhythmia, and Ischemic Heart Disease), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033.
Industry: HealthcareGlobal Cardiovascular Clinical Trials Market Insights Forecasts to 2033
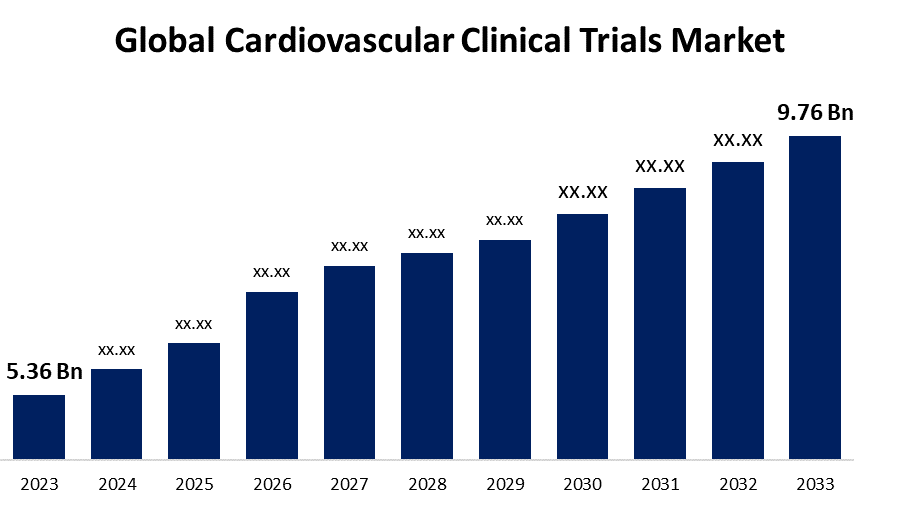
- The Global Cardiovascular Clinical Trials Market Size was Valued at USD 5.36 Billion in 2023
- The Market Size is Growing at a CAGR of 6.18% from 2023 to 2033
- The Worldwide Cardiovascular Clinical Trials Market Size is Expected to Reach USD 9.76 Billion by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Cardiovascular Clinical Trials Market Size is Anticipated to Exceed USD 9.76 Billion by 2033, Growing at a CAGR of 6.18% from 2023 to 2033.
Market Overview
Cardiovascular clinical trials are methodical studies carried out on human participants to evaluate the general impact, safety, and effectiveness of novel medications, medical technologies, or therapies pertaining to cardiovascular health. Strict ethical and scientific guidelines are followed during these trials to guarantee the validity of the results. By examining the prevention, diagnosis, and treatment of cardiovascular disorders, such as heart failure, coronary artery disease, and stroke, these trials hope to further medical understanding and enhance patient outcomes. Clinical trials are conducted in four phases, including phase I, phase II, phase III, and phase IV. These trials are carried out in stages by researchers, who begin with a smaller sample to evaluate safety and then progressively expand to a wider population to collect more detailed information on efficacy and possible adverse effects. The increase in cardiovascular clinical trials is anticipated to investigate novel therapies and treatment approaches that support the growth of the market. To assess clinical outcomes, the Mayo Clinic, for example, is running a clinical trial on a superficially, in order to address the necessary changes to lessen the burden of cardiovascular diseases, advocacy and ongoing awareness are needed, as is the ability to predict market opportunities for market participants. cervical plexus block for pacemaker installation. Consequently, in order to address the necessary changes to lessen the burden of cardiovascular diseases, advocacy and ongoing awareness are needed, as is the ability to predict market opportunities for market participants.
Report Coverage
This research report categorizes the market for the global cardiovascular clinical trials market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global cardiovascular clinical trials market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global cardiovascular clinical trials market.
Global Cardiovascular Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 5.36 Billion |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 6.18% |
| 2033 Value Projection: | USD 9.76 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Phase, By Study Design, By Region |
| Companies covered:: | ICON plc, Eli Lilly and Company, Syneos Health, Worldwide Clinical Trials, Veeda Clinical Research, IQVIA Inc, SGS SA, PPD Inc, Caidya, Vial, Medpace, Inc., Thermo Fisher Scientific, Merck & Co, Pfizer, Others |
| Pitfalls & Challenges: | Covid 19 Impact Challanges, Future, Growth and Analysis |
Get more details on this report -
Driving Factors
The primary driver of the growth in the cardiovascular clinical trials market is the rising incidence of cardiovascular conditions such as heart failure, stroke, and coronary artery disease. The increasing awareness of cardiovascular health issues and their impact on global morbidity and mortality rates acts as a primary driver for the expansion of cardiovascular clinical trials. There is a growing demand for tailored and patient-specific treatment approaches, encouraging researchers to conduct trials that explore individualized interventions based on genetic, lifestyle, and biomarker considerations. These factors drive the growth of the cardiovascular clinical trials market.
Restraining Factors
Cardiovascular clinical trials phase ongoing challenges in enrolling a diverse and representative patient population and maintaining their participation throughout the trial duration. These issues are compounded by the complex regulatory landscape and strict compliance requirements that face market players and affect the time and resources needed for trial initiation and completion. These factors restrain the growth of the cardiovascular clinical trials market.
Market Segmentation
The global cardiovascular clinical trials market share is classified into phase, study design, and indication.
- The phase IV segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period.
On the basis of the phase, the global cardiovascular clinical trials market is divided into phase I, phase II, phase III, and phase IV. Among these, the phase IV segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period. To guarantee the continuous efficacy and safety of cardiovascular medicines, regulatory organizations like the European Union regulatory authorities and the U.S. Food and Drug Administration may mandate post-marketing studies. Phase 4 trials are conducted based on adherence to these guidelines. Furthermore, there is a growing need to investigate the advantages of incorporating real-world data into clinical trials due to the explosion of patient data and advancements in genomics. Moreover, real-world data is most frequently incorporated with phase IV clinical trials for payer approvals and health technology assessments, according to industry experts.
- The interventional segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period.
On the basis of the study design, the global cardiovascular clinical trials market is divided into interventional, observational, and expanded access. Among these, the interventional segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period. The interventional segment is further classified into drugs or biologics, behavioral procedures, surgical procedures, and devices. Since interventional studies are more accurate and relevant than observational research, they are typically chosen. Furthermore, interventional studies are the gold standard for determining the precision and effectiveness of medical devices under investigation in cardiovascular medical device clinical trials, which helps to explain the large market share of the studied products.
- The coronary artery disease segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period.
On the basis of the indication, the global cardiovascular clinical trials market is divided into coronary artery disease, cardiac arrhythmia, and ischemic heart disease. Among these, the coronary artery disease segment is anticipated to hold the largest share of the global cardiovascular clinical trials market during the forecast period. The reason for this is the increasing number of clinical trials related to coronary artery disease that are being evaluated. This, in turn, is proportionate to the increasing prevalence rate of the condition worldwide.
Regional Segment Analysis of the Global Cardiovascular Clinical Trials Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
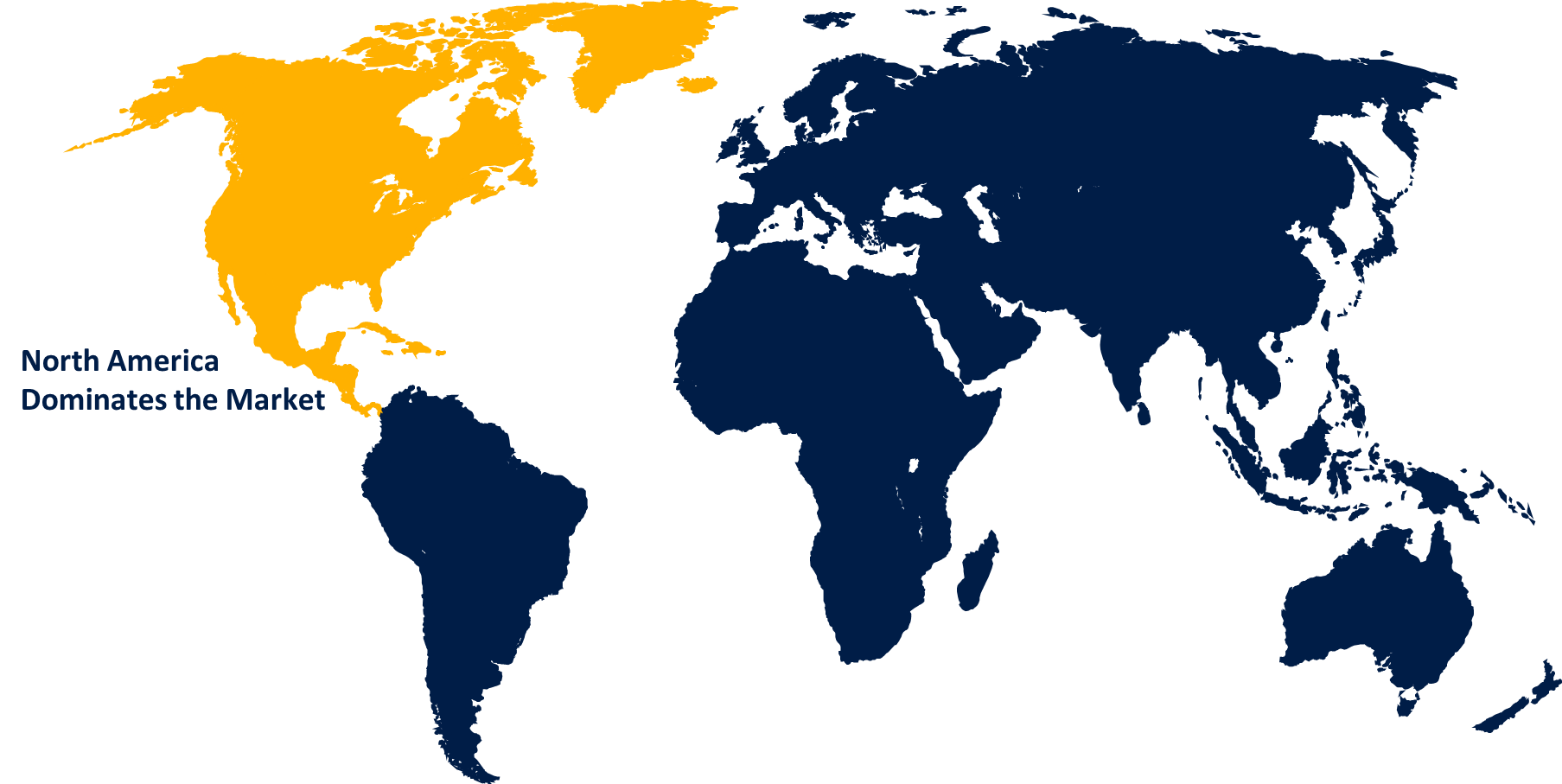
North America is anticipated to hold the largest share of the global cardiovascular clinical trials market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global cardiovascular clinical trials market over the predicted timeframe. The largest market for cardiovascular clinical trials is the United States. With the implementation of several innovative, ground-breaking technologies in clinical research and the provision of state-of-the-art infrastructure, numerous clinical trials for heart disorders are being done countrywide. For example, according to Clinicaltrials.gov, there were around 5,068 registered cardiovascular clinical studies in the United States as of September 2023. Therefore, it is expected that the previously described factors will sustain the majority of the North American region.
Asia-Pacific is anticipated to grow at the fastest rate in the global cardiovascular clinical trials market over the predicted timeframe. The cost-effective clinical research alternatives and favorable regulatory reforms, particularly in China and India, Asia-Pacific is one of the most appealing markets for conducting clinical trials. Furthermore, the Association of Standardized Patient Educators reports that a number of factors, including lower costs and shorter schedules that make conducting trials outside of the United States easier and less expensive, are causing the clinical research footprint to shift to developing economies. For example, the National Center for Biotechnology Information reports that in 2022, clinical trial participation from foreign pharmaceutical companies will have grown in India due to the country's greater capacity to attract patients and quickly lower trial costs.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global cardiovascular clinical trials market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- ICON plc
- Eli Lilly and Company
- Syneos Health
- Worldwide Clinical Trials
- Veeda Clinical Research
- IQVIA Inc
- SGS SA
- PPD Inc
- Caidya
- Vial
- Medpace, Inc.
- Thermo Fisher Scientific
- Merck & Co
- Pfizer
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In October 2023, the Icahn School of Medicine at Mount Sinai has organized an agreement with the Chiba Institute of Technology (CIT) in partnership to explore the application of artificial intelligence (AI) in cardiovascular disorder research. The purpose of the agreement is to increase the efficiency of clinical trials, accelerate progress in patient care, and promote the possible introduction of new treatments for heart patients.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global cardiovascular clinical trials market based on the below-mentioned segments:
Global Cardiovascular Clinical Trials Market, By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Global Cardiovascular Clinical Trials Market, By Study Design
- Interventional
- Observational
- Expanded Access
Global Cardiovascular Clinical Trials Market, By Indication
- Coronary Artery Disease
- Cardiac Arrhythmia
- Ischemic Heart Disease
Global Cardiovascular Clinical Trials Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. Which phase segment have the largest share in the global cardiovascular clinical trials market?1. Which phase segment have the largest share in the global cardiovascular clinical trials market?
-
2. How big is global cardiovascular clinical trials market?The Global Cardiovascular Clinical Trials Market Size is Expected to Grow from USD 5.36 Billion in 2023 to USD 9.76 Billion by 2033, at a CAGR of 6.18% during the forecast period 2023-2033.
-
3. What are the factors driving the global cardiovascular clinical trials market?The primary driver factors of the growth in the cardiovascular clinical trials market are the rising incidence of cardiovascular conditions such as heart failure, stroke, and coronary artery disease, the increasing awareness of cardiovascular health issues, and their impact on global morbidity and mortality rate.
Need help to buy this report?