Global Endotoxin Testing Market Size, Share, and COVID-19 Impact Analysis, By Technology (Gel Clot Endotoxin Testing, Chromogenic Endotoxin Testing, Turbidimetric Endotoxin Testing, and Others), By End Users (Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs), and Medical Device Manufacturers), and By Region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Endotoxin Testing Market Insights Forecasts to 2033
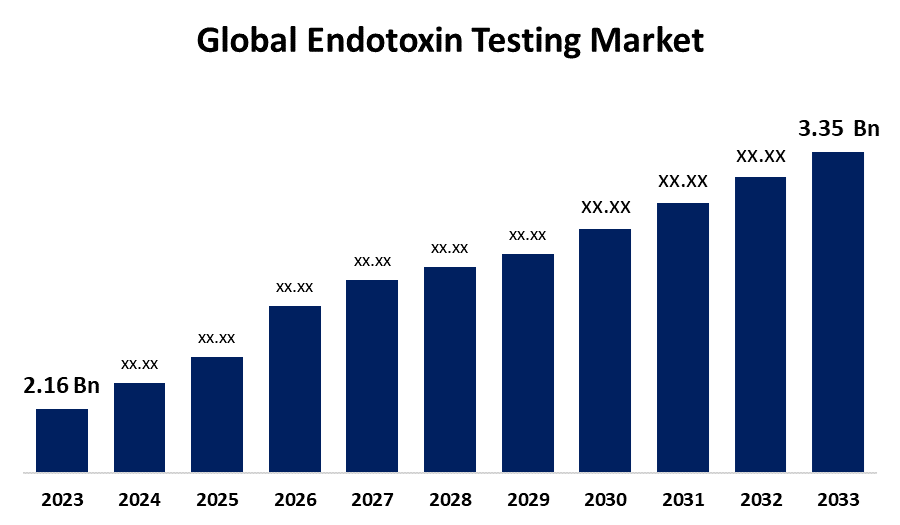
- The Global Endotoxin Testing Market Size was Valued at USD 2.16 Billion in 2023
- The Market Size is Growing at a CAGR of 4.49% from 2023 to 2033
- The Worldwide Endotoxin Testing Market Size is Expected to Reach USD 3.35 Billion by 2033
- Europe is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Endotoxin Testing Market Size is anticipated to exceed USD 3.35 Billion by 2033, growing at a CAGR of 4.49% from 2023 to 2033. The global endotoxin testing market is growing at a rapid pace due to the increasing demand for pharmaceuticals, biopharmaceuticals, and medical devices. Advancements in testing technologies and stringent regulations in healthcare sectors are major factors driving the growth of this market.
Market Overview
Endotoxin testing, also known as the Bacterial Endotoxins Test (BET), is an in vitro procedure used to measure the amounts of bacterial endotoxins in substances, especially medications and medical equipment, in order to guarantee product safety and adherence to quality standards. This examination aids in avoiding any negative consequences for users and patients. Additionally, future growth in the endotoxin testing market is anticipated to be driven by the rising incidence of infections linked to healthcare. Infections contracted during medical treatment that were not present at the time of hospital admission are referred to as healthcare-associated infections. Thorough testing is necessary to guarantee patient safety since healthcare-associated illnesses frequently result from contaminated medical procedures or equipment.
Report Coverage
This research report categorizes the global endotoxin testing market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global endotoxin testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global endotoxin testing market.
Global Endotoxin Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023 : | 2.16 Billion |
| Forecast Period: | 2023 – 2033 |
| Forecast Period CAGR 2023 – 2033 : | 4.49% |
| 023 – 2033 Value Projection: | 3.35 Billion |
| Historical Data for: | 2021-2022 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 111 |
| Segments covered: | By Technology, By End Users and By Region, |
| Companies covered:: | Thermo Fisher Scientific Inc., Merck KGaA, bioMérieux SA, Eurofins Scientific SE, Lonza Group Ltd., WuXi AppTec Co. Ltd., Bio-Rad Laboratories Inc., Maravai LifeSciences Holdings Inc., Cambrex Corporatio, GenScript Biotech Corporation, Charles River Laboratories International Inc., Nelson Laboratories LLC, Others, |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market for endotoxin testing is expanding due in large part to the growing demand for endotoxin testing worldwide, as demonstrated by bioMérieux, which reports that between 70 million and 100 million tests are performed globally each year. Additionally, in May 2022, 52.3% of patients treated in intensive care units die annually, and 24% of patients suffer from healthcare-associated sepsis, according to the World Health Organization, a United Nations body based in Switzerland. The significant occurrence of sepsis, particularly in intensive care units, emphasizes how crucial prompt and precise endotoxin testing is to patient safety and efficient infection control. The market for endotoxin testing is expanding as a consequence of the rising demand for endotoxin testing services and products due to the growing requirement for accurate testing techniques. Furthermore, the market for endotoxin and pyrogen testing has a lot of opportunities because to improvements in automated and rapid testing technology. These developments increase productivity, precision, and capacities, satisfying the increasing demand for strict quality control in the production of pharmaceuticals and medical devices and so driving market expansion.
Restraints & Challenges
The endotoxin testing market's expansion is being restrained during the forecast period by the high cost of treatments. Exorbitant treatment expenses may lower the demand for endotoxin testing as well as other medical products and therapies.
Market Segmentation
The global endotoxin testing market share is classified into technology and end users.
- The gel clot endotoxin testing segment is expected to hold the largest share of the global endotoxin testing market during the forecast period.
Based on the technology, the global endotoxin testing market is categorized as gel clot endotoxin testing, chromogenic endotoxin testing, turbidimetric endotoxin testing, and others. Among these, the gel clot endotoxin testing segment is expected to hold the largest share of the global endotoxin testing market during the forecast period.Gel clot technique is still often used for routine testing since it is affordable and simple to use. In automated and high-throughput environments, turbidimetric and chromogenic procedures are used because they yield quantitative data with great sensitivity.
- The pharmaceutical and biotechnology companies’ segment is expected to grow at the fastest CAGR during the forecast period.
Based on the end users, the global endotoxin testing market is categorized as pharmaceutical and biotechnology companies, contract research organizations (CROs), and medical device manufacturers. Among these, the pharmaceutical and biotechnology companies’ segment is expected to grow at the fastest CAGR during the forecast period. This is due to the growing manufacturing of drugs and biologics, which need stringent endotoxin testing to guarantee safety and compliance, pharmaceutical and biotechnology firms consider a major proportion.
Regional Segment Analysis of the Global Endotoxin testing Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
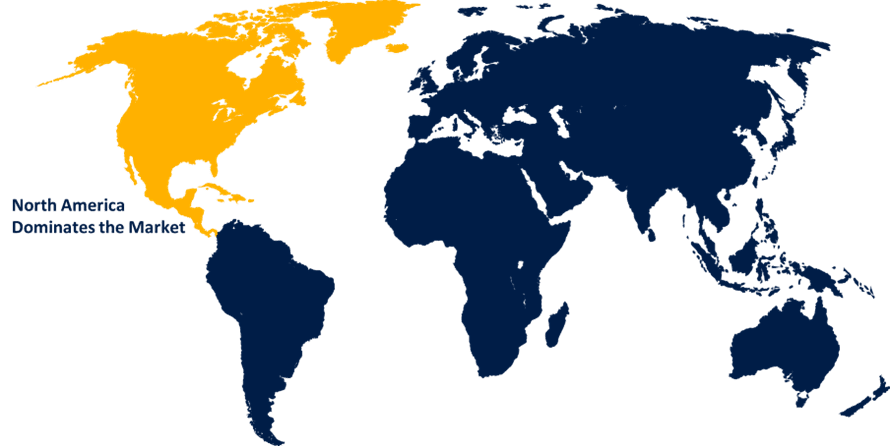
North America is projected to hold the largest share of the global endotoxin testing market over the forecast period.
Get more details on this report -
North America is projected to hold the largest share of the global endotoxin testing market over the forecast period. The existence of a firmly established biotechnology and pharmaceutical industry as well as strict regulatory frameworks implemented by organizations like the U.S. Food and Drug Administration (FDA) are responsible for this supremacy. The market is driven by the region's strong healthcare system, substantial investment in drug discovery, and high demand for biologics and medical devices.
Europe is expected to grow at the fastest CAGR growth of the global endotoxin testing market during the forecast period. Strict endotoxin testing guidelines are required by the European Medicines Agency (EMA) to guarantee product safety and compliance. Germany, France, and the UK are important nations driving the market's expansion due to their high biopharmaceutical investments and increasing focus on natural and recombinant endotoxin testing alternatives.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global endotoxin testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Thermo Fisher Scientific Inc.
- Merck KGaA
- bioMérieux SA
- Eurofins Scientific SE
- Lonza Group Ltd.
- WuXi AppTec Co. Ltd.
- Bio-Rad Laboratories Inc.
- Maravai LifeSciences Holdings Inc.
- Cambrex Corporatio
- GenScript Biotech Corporation
- Charles River Laboratories International Inc.
- Nelson Laboratories LLC
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Key Market Developments
- In January 2022, Sentinel Monitoring Systems Inc. was purchased by US-based water treatment business SUEZ Water Technologies and Solutions for an unknown sum. This acquisition creates additional market opportunities for microbiological monitoring and expands the line of analytical instruments offered by Veolia Water Technologies & Solutions.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global endotoxin testing market based on the below-mentioned segments:
Global Endotoxin Testing Market, By Technology
- Gel Clot Endotoxin Testing
- Chromogenic Endotoxin Testing
- Turbidimetric Endotoxin Testing
- Others
Global Endotoxin Testing Market, By End Users
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations (CROs)
- Medical Device Manufacturers
Global Endotoxin Testing Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the global endotoxin testing market over the forecast period?The global endotoxin testing market size is expected to grow from USD 2.16 Billion in 2023 to USD 3.35 Billion by 2033, at a CAGR of 4.49% during the forecast period 2023-2033.
-
2. Which region is expected to hold the highest share of the global endotoxin testing market?North America is projected to hold the largest share of the global endotoxin testing market over the forecast period.
-
3. Who are the top key players in the global endotoxin testing market?Thermo Fisher Scientific Inc., Merck KGaA, bioMérieux SA, Eurofins Scientific SE, Lonza Group Ltd., WuXi AppTec Co. Ltd., Bio-Rad Laboratories Inc., Maravai LifeSciences Holdings Inc., Cambrex Corporation, GenScript Biotech Corporation, Charles River Laboratories International Inc., Nelson Laboratories LLC, and Others.
Need help to buy this report?