Europe Cosmetovigilance Market Size, Share, and COVID-19 Impact Analysis, By Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), By Services Type (Pre-Marketing and Post-Marketing), and Europe Cosmetovigilance Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareEurope Cosmetovigilance Market Insights Forecasts to 2033
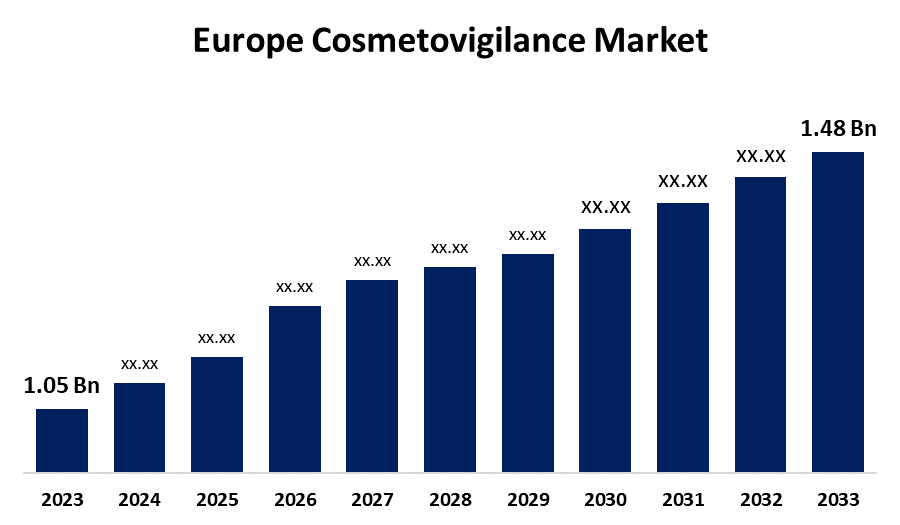
- The Europe Cosmetovigilance Market Size was valued at USD 1.05 Billion in 2023.
- The Market Size is Growing at a CAGR of 3.49% from 2023 to 2033
- The Europe Cosmetovigilance Market Size is Expected to Reach USD 1.48 Billion by 2033
Get more details on this report -
The Europe Cosmetovigilance Market Size is Anticipated to Reach USD 1.48 Billion by 2033, Growing at a CAGR of 3.49% from 2023 to 2033
Market Overview
Cosmetovigilance is the continuous compilation, evaluation, and monitoring of cosmetic products’ safety, concerning human health. Manufacturers have the responsibility to determine that cosmetic products and their ingredients are safe before they enter the market, as well as to gather reports of adverse reactions. Additionally, In the European Union (EU), cosmetovigilance is established under the Cosmetics Regulation (EC) No. 1223/2009. As stated in this Regulation, “ensuring traceability of a cosmetic product throughout the whole supply chain helps to make market surveillance simpler and more efficient”. According to the EU Cosmetics Regulation (EC) No. 1223/2009, a Product Information File (PIF) for each cosmetic product must be prepared by a safety assessor (qualified person) before the product is placed on the market. This PIF shall be made readily accessible to the competent authority of the Member State (at one single address within the Community). Furthermore, The Europe cosmetovigilance market is primarily driven by strict regulations requiring thorough safety monitoring of cosmetics.
Report Coverage
This research report categorizes the market for the Europe cosmetovigilance market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Europe cosmetovigilance market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Europe cosmetovigilance market.
Europe Cosmetovigilance Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 1.05 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 3.49% |
| 2033 Value Projection: | USD 1.48 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 166 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Phase, By Services Type |
| Companies covered:: | ProClinical, Skillpharma, Di Renzo Regulatory Affairs, Aixial Group, PharSafer, AllianceBernstein Holding LP, Poseidon Expeditions, Accenture PLC Class A, and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The cosmetovigilance market is driven by several factors including, consumers becoming more aware of product safety and reporting adverse reactions to regulatory authorities. Manufacturers are required to report adverse events. Ensuring product safety is important to maintain a brand reputation. Allergic reactions to cosmetics are becoming more prevalent due to new ingredients, formulations, and fragrances. Regulatory agencies are imposing stricter regulations and surveillance measures on cosmetic manufacturers.
Restraining Factors
Several factors act as a restrain to the growth of the Europe cosmetovigilance market include, the high cost of executing and maintaining cosmetovigilance systems is high. Underdeveloped countries may lack the skilled professionals needed for cosmetovigilance. Middle and lower-income countries may lack standardized reporting systems and definitions for adverse events. Some regions may lack comprehensive regulatory requirements for cosmetovigilance practices. Developing and underdeveloped countries may lack awareness of cosmetovigilance.
Market Segmentation
The Europe cosmetovigilance market share is classified into phase and service type.
- The phase IV segment is expected to hold the largest market share through the forecast period.
The Europe cosmetovigilance market is segmented by phase into pre-clinical, phase I, phase II, phase III, and phase IV. Among these, the phase IV segment is expected to hold the largest market share through the forecast period. These phases serve as an extra layer of safety for the cosmetics undergoing clinical trials. Phase IV of clinical trials is crucial because it allows for the identification of potential adverse medication reactions. Due to comprehensive drug testing on a large patient base, the data gathered and evaluated during this phase is thus expected to be of the utmost relevance following the commercialization of the product.
- The post-marketing segment is expected to dominate the Europe cosmetovigilance market during the forecast period.
Based on the service type, the Europe cosmetovigilance market is divided into pre-marketing and post-marketing. Among these, the post-marketing segment is expected to dominate the Europe cosmetovigilance market during the forecast period. Owing to the expanded need for cosmetovigilance services, to curb unfavorable effects (UEs) and substantial unfavorable effects (SUEs) associated with the use of cosmetics. Additionally, the process of analyzing and reporting the adverse reactions related to cosmetic products is primarily driven by the cosmetics industry. Furthermore, along with the adverse reaction reporting, post-marketing services also include case intake, case triage, and data entry and acquisition, propelling the segment growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Europe cosmetovigilance market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- ProClinical
- Skillpharma
- Di Renzo Regulatory Affairs
- Aixial Group
- PharSafer
- AllianceBernstein Holding LP
- Poseidon Expeditions
- Accenture PLC Class A
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In March 2024, Accenture agreed to acquire Arηs Group, a technology services provider focused on supporting public sector transformation across Europe. Arηs Group has widespread experience in helping drive modernization projects for European institutions.
Market Segment
This study forecasts revenue at Europe, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Europe Cosmetovigilance Market based on the below-mentioned segments:
Europe Cosmetovigilance Market, By Phase
- Pre-Clinical
- Phase I
- Phase II
- Phase III
- Phase IV
Europe Cosmetovigilance Market, By Service Type
- Pre-Marketing
- Post-Marketing
Need help to buy this report?