Europe Pharmaceutical Contract Research Organization (CRO) Market Size, Share, and COVID-19 Impact Analysis, By Type (Drug Discovery, Pre-Clinical, and Clinical), By Service (Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/ Assurance, Bio-statistics, and Others), and Europe Pharmaceutical Contract Research Organization (CRO) Market Insights, Industry Trend, Forecasts to 2033.
Industry: HealthcareEurope Pharmaceutical Contract Research Organization (CRO) Market Insights Forecasts to 2033
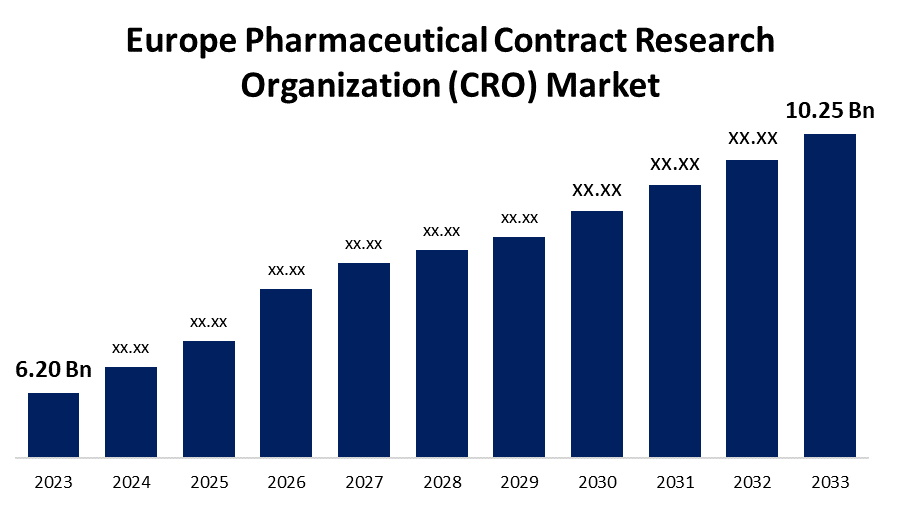
- The Europe Pharmaceutical Contract Research Organization (CRO) Market Size was valued at USD 6.20 Billion in 2023.
- The Market Size is Growing at a CAGR of 5.16% from 2023 to 2033
- The Europe Pharmaceutical Contract Research Organization (CRO) Market Size is Expected to Reach USD 10.25 Billion by 2033
Get more details on this report -
The Europe Pharmaceutical Contract Research Organization (CRO) Market Size is Anticipated to Reach USD 10.25 Billion by 2033, growing at a CAGR of 5.16% from 2023 to 2033.
Market Overview
A pharmaceutical Contract Research Organization (CRO) is a business that offers research and development (R&D) services to pharmaceutical firms. CROs are contracted to oversee and execute clinical trials, which are essential in the drug development process. Companies opt to collaborate with CROs due to their expertise and experience required for managing clinical trials. By outsourcing clinical trial responsibilities to a CRO, companies can conserve time and resources while concentrating on other critical activities. CROs in Europe offer a comprehensive range of services, including clinical trial design, patient enrollment, site supervision, data evaluation, regulatory guidance, biostatistics, medical writing, and pharmacovigilance. Multiple large global CROs have a significant presence in Europe, in addition to many regional organizations that focus on specific therapeutic areas or stages of clinical trials. Nations such as Germany, France, the UK, Spain, and Switzerland are recognized as major centers for clinical trials, due to their well-established research infrastructure and ample patient demographics. The European pharmaceutical CRO market, fueled by the high incidence of chronic conditions like cancer, Alzheimer’s disease, heart disease, and others, has been growing rapidly across Europe. Europe is home to numerous reputable research institutions, medical centers, and hospitals, which provide a robust foundation for generating and advancing scientific and clinical innovations.
Report Coverage
This research report categorizes the market for the Europe pharmaceutical contract research organization (CRO) based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Europe pharmaceutical contract research organization (CRO) market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Europe pharmaceutical contract research organization (CRO) market.
Europe Pharmaceutical Contract Research Organization (CRO) Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 6.20 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 5.16% |
| 2033 Value Projection: | USD 10.25 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 156 |
| Segments covered: | By Type, By Service |
| Companies covered:: | IQVIA, Worldwide Clinical Trials, Syneos Health, Medpace, Clinipace, WuXi AppTec, Almac, FGK Clinical Research, CTI, and other key companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The European pharmaceutical CRO market is mainly driven by the increasing prevalence of chronic illnesses such as cancer and heart disease, resulting in a heightened demand for effective treatments. At the same time, there is a growing trend among pharmaceutical companies to outsource research and development activities to CROs, motivated by cost-effectiveness and access to specialized knowledge; this trend includes a rise in clinical trials alongside the necessity to handle complex drug development processes. The growing rates of chronic conditions, including cancer, diabetes, and cardiovascular diseases, are elevating the demand for new treatment alternatives, which in turn boosts the need for CRO services to manage clinical trials and drug development. Pharmaceutical firms are progressively outsourcing R&D tasks to CROs to lower expenses, tap into specialized knowledge, and hasten the drug development timeline. The increasing intricacies of drug development, particularly in areas like personalized medicine and targeted therapies, require CROs equipped with advanced technologies and expertise to administer clinical trials proficiently.
Restraining Factors
Several key hindering factors for the European pharmaceutical CRO market consist of a lack of skilled personnel, stringent regulatory adherence, high expenses associated with clinical trials, intricate clinical trial designs, fierce competition among CROs, concerns regarding data privacy, and the challenge of navigating various national regulations throughout Europe; all of these factors may impede market growth by affecting operational efficiency and escalating costs for CROs.
Market Segmentation
The Europe pharmaceutical contract research organization (CRO) market share is classified into type and service.
- The clinical segment is expected to hold the largest market share through the forecast period.
The Europe pharmaceutical contract research organization (CRO) market is segmented by type into drug discovery, pre-clinical, and clinical. Among these, the clinical segment is expected to hold the largest market share through the forecast period. The increase in this segment can be attributed to the rising need for clinical trials, a strict regulatory framework, and a transition towards a patient-centered approach. Contract Research Organizations (CROs) are adopting technologies and methodologies to improve patient engagement and retention, which further solidifies their position in the clinical sector.
- The clinical monitoring segment is expected to dominate the Europe pharmaceutical contract research organization (CRO) market during the forecast period.
Based on the service, the Europe pharmaceutical contract research organization (CRO) market is divided into project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/ assurance, bio-statistics, and others. Among these, the clinical monitoring segment is expected to dominate the Europe pharmaceutical contract research organization (CRO) market during the forecast period. Clinical monitoring plays a vital role in maintaining the integrity and compliance of clinical trials. It manages the execution of clinical trials, confirms the accuracy of data, and ensures compliance with regulatory standards, thus serving as a key element in the drug development process.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Europe pharmaceutical contract research organization (CRO) market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA
- Worldwide Clinical Trials
- Syneos Health
- Medpace
- Clinipace
- WuXi AppTec
- Almac
- FGK Clinical Research
- CTI
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In March 2024, Veeda Clinical Research Limited, a comprehensive contract research organization (CRO) known for its successful drug development track record, has announced its acquisition of Heads, a privately owned European CRO that focuses on carrying out clinical trials in the field of oncology.
Market Segment
This study forecasts revenue at Europe, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Europe Pharmaceutical Contract Research Organization (CRO) Market based on the below-mentioned segments
Europe Pharmaceutical Contract Research Organization (CRO) Market, By Type
- Drug Discovery
- Pre-Clinical
- Clinical
Europe Pharmaceutical Contract Research Organization (CRO) Market, By Services
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Others
Need help to buy this report?