Europe Radiodermatitis Market Size, Share, and COVID-19 Impact Analysis, By Product (Topical, Oral, and Dressings), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online), and Europe Radiodermatitis Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareEurope Radiodermatitis Market Insights Forecasts to 2033
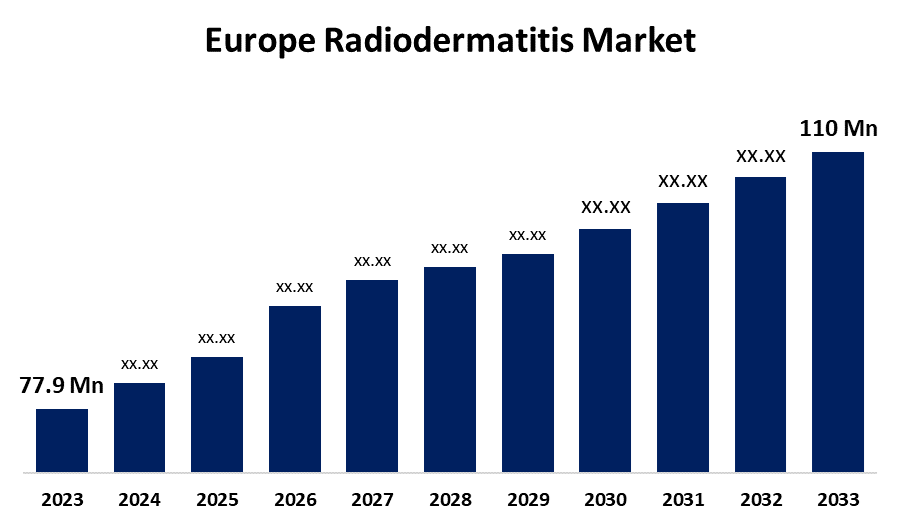
- The Europe Radiodermatitis Market Size was valued at USD 77.9 Million in 2023
- The Market is Growing at a CAGR of 3.51% from 2023 to 2033
- The Europe Radiodermatitis Market Size is Expected to Reach USD 110 Million by 2033
Get more details on this report -
The Europe Radiodermatitis Market Size is Anticipated to Reach USD 110 million by 2033, Growing at a CAGR of 3.51% from 2023 to 2033.
Market Overview
Radiodermatitis, also known as radiation dermatitis, is a skin condition that occurs as a result of exposure to ionizing radiation. It can be a side effect of radiation therapy for cancer, nuclear attacks, or other disasters. Radiodermatitis can cause the skin to become painful, red, itchy, and blistered. It can be acute or chronic, depending on when the skin reaction occurs. Radiodermatitis (RD) is a common side effect of radiotherapy and affects patients in Europe. The European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG) have a standardized grading system for acute radiation-induced skin toxicity. The Radiation-Induced Skin Reaction Assessment Scale (RISRAS) is a tool that helps evaluate ARD using patient symptoms and healthcare professional assessment scales. There are several guidelines for managing radiodermatitis, including those from the Association Francophone des Soins Oncologiques de Support, the Multinational Association of Supportive Care in Cancer, and European Skin Management in Oncology. Furthermore, the need to improve the quality of life for cancer patients and the use of a combination of chemotherapy and radiation therapy further fuel the growth of the European radiodermatitis market.
Report Coverage
This research report categorizes the market for the Europe radiodermatitis based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Europe radiodermatitis market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Europe radiodermatitis market.
Europe Radiodermatitis Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 77.9 Million |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 3.51% |
| 2033 Value Projection: | USD 110 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 219 |
| Tables, Charts & Figures: | 99 |
| Segments covered: | By Product, By Distribution Channel and COVID-19 Impact Analysis. |
| Companies covered:: | BMG Pharma, Stratpharma, Alliqua BioMedical, Molnlycke Health Care, Integra LifeSciences, ConvaTec Group PLC, Smith & Nephew PLC, 3M Co, and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The radiodermatitis market in Europe is driven by several factors. One major driver is the increase in the prevalence of cancer globally. Another key factor is the rising adoption of radiotherapy as a cancer treatment. Additionally, the growing number of older people receiving radiation therapy contributes to the growth of the radiodermatitis market. Moreover, the rising cost of healthcare in both developed and developing countries plays a role in the market's expansion. Favorable government policies and reimbursement plans for cancer treatments and their side effects also contribute to the growth of the radiodermatitis market.
Restraining Factors
Several factors could limit the growth of the radiodermatitis market in Europe. The high cost of radiodermatitis treatments, particularly dressings, can restrict market expansion. New radiodermatitis treatments must undergo rigorous regulatory reviews, which can be time-consuming and expensive. Additionally, more clinical evidence is necessary to confirm the safety and effectiveness of certain radiodermatitis treatments. Patients and healthcare professionals might prefer alternative therapies and natural remedies due to cost, perceived safety, or cultural preferences.
Market Segmentation
The Europe radiodermatitis market share is classified into product and distribution channels.
- The topical segment is expected to hold the largest market share through the forecast period.
The Europe radiodermatitis market is segmented by product into topical, oral, and dressings. Among these, the topical segment is expected to hold the largest market share through the forecast period. A significant portion is thought to result from the advantages of topical products. These advantages, which are some of the primary driving forces for the acceptance of these items throughout the previous years, include ease of use, accessibility, and great cost-efficiency. Additionally, topical products lessen the chance of microbiological transmission and protect the skin from abrasive substances. Some of the key items under this category are topical antibiotics, hydrophilic lotions, and corticosteroids. Due to their reputation as the gold standard in the treatment of inflammatory skin disorders, which prompted their adoption for the treatment of radiodermatitis, corticosteroids accounted for the biggest proportion among topical agents.
- The retail pharmacies segment is expected to dominate the Europe radiodermatitis market during the forecast period.
Based on the distribution channel, the Europe radiodermatitis market is divided into hospital pharmacies, retail pharmacies, and online. Among these, the retail pharmacies segment is expected to dominate the Europe radiodermatitis market during the forecast period. The significant percentage can be attributable to retail stores' high accessibility and cost. Drug cost reimbursement encourages patients to rely on retail pharmacies, which increases market growth potential as a result of affordability.Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Europe radiodermatitis market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- BMG Pharma
- Stratpharma
- Alliqua BioMedical
- Molnlycke Health Care
- Integra LifeSciences
- ConvaTec Group PLC
- Smith & Nephew PLC
- 3M Co
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Europe, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Europe Radiodermatitis Market based on the below-mentioned segments:
Europe Radiodermatitis Market, By Product
- Topical
- Oral
- Dressings
Europe Radiodermatitis Market, By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online
Need help to buy this report?