Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market Size, Share, and COVID-19 Impact Analysis, By Product Type (XLIF Interbody Fusion Systems and XLIF Interbody Cages), By Application (Hospitals, Ambulatory Surgical Centers, and Orthopedic Clinics), and By Region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Extreme lateral interbody fusion (XLIF) surgery Market Insights Forecasts to 2033
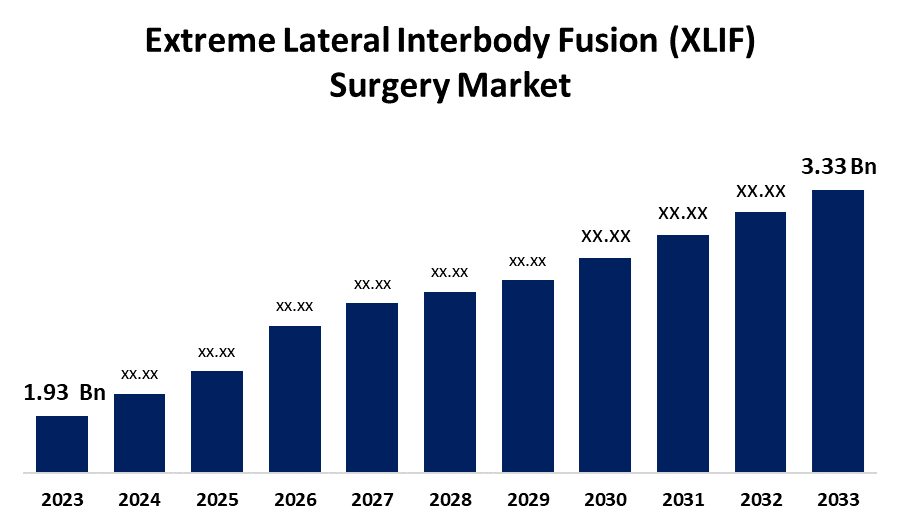
- The Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market Size was Valued at USD 995.29 Million in 2023
- The Market Size is Growing at a CAGR of 4.41% from 2023 to 2033
- The Worldwide Extreme Lateral Interbody Fusion (XLIF) Surgery Market Size is Expected to Reach USD 1532.19 Million by 2033
- Europe is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market Size is anticipated to exceed USD 1532.19 Million by 2033, growing at a CAGR of 4.41% from 2023 to 2033.
Market Overview
In lumbar spinal fusion surgery, also known as lumbar interbody fusion, the neurosurgeon enters the spine from the side of the body using a minimally invasive technique known as extreme lateral interbody fusion, or XLIF for short. It is a surgical procedure that is utilized to treat several spine conditions. The damaged area is fused during the procedure, which stabilizes the spine and reduces any pain that may be present. Specialized surgical equipment is used for XLIF procedures, and the participating medical experts must take advantage of imaging tools. To remove damaged or deteriorated discs between the vertebrae, the surgeon creates a little incision on the patient's side to provide access to the spine. Additionally, the growing number of elderly generations, who are more vulnerable to chronic illnesses, is another factor driving this development. Patients and healthcare professionals are drawn to XLIF surgery because of its minimally invasive nature, which provides advantages like less postoperative pain, shorter hospital stays, and faster recovery times.
Report Coverage
This research report categorizes the global extreme lateral interbody fusion (XLIF) surgery market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global extreme lateral interbody fusion (XLIF) surgery market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global extreme lateral interbody fusion (XLIF) surgery market.
Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023 : | 1.93 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 5.61% |
| 2033 Value Projection: | 3.33 Billion |
| Historical Data for: | 2021-2022 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 106 |
| Segments covered: | By Product Type, By Application and By Region |
| Companies covered:: | Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet Holdings Inc., NuVasive Inc., Globus Medical Inc., DePuy Synthes, B. Braun Melsungen AG, Alphatec Holdings Inc., RTI Surgical Holdings Inc., K2M Group Holdings Inc., Orthofix Medical Inc., Aesculap Implant Systems, SpineWave Inc., Others, |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rising incidence of spinal illnesses worldwide is expected to fuel growth in the market for extreme lateral interbody fusion (XLIF) surgery. The general population's sedentary lifestyle is contributing to the rise in conditions like spinal stenosis, degenerative disc disease, and ruptured discs. DSD and LBP affect 266 million people (3.63%) globally each year, with Europe having the highest incidence (5.7%) and Africa having the lowest (2.4%). The need for cutting-edge surgical techniques like Extreme Lateral Interbody Fusion (XLIF) is driven by the rising incidence of spinal disorders, which helps to boost the growth of the worldwide XLIF surgery market. Furthermore, about 39 million people worldwide are afflicted with spondylolisthesis, which is defined as the forward displacement of a vertebra; this represents an incidence rate of 0.53% annually. The market is expanding as a consequence of the growing number of patients with spondylolisthesis, which necessitates Extreme Lateral Interbody Fusion (XLIF) surgery. The market is expanding as an outcome of healthcare providers being encouraged to invest in and implement XLIF procedures due to the spike in demand.
Restraints & Challenges
When compared to alternative medical procedures used to treat spinal cord-related conditions, XLIF surgery is more expensive. The cost of therapy and aftercare may be prohibitive for many lower-income households in a growing nation like India.
Market Segmentation
The global extreme lateral interbody fusion (XLIF) surgery market share is classified into product type and application.
- The XLIF interbody cages segment is expected to hold the largest share of the global extreme lateral interbody fusion (XLIF) surgery market during the forecast period.
Based on product type, the global extreme lateral interbody fusion (XLIF) surgery market is categorized as XLIF interbody fusion systems and XLIF interbody cages. Among these, the XLIF interbody cages segment is expected to hold the largest share of the global extreme lateral interbody fusion (XLIF) surgery market during the forecast period. XLIF interbody cages are implantable devices made especially for interbody fusion during XLIF procedures. In order to assist fusion, decompress nerves, and restore disc height, they are positioned between neighboring vertebrae. These cages are utilized more frequently because they are regarded as the main part of the process.
- The hospitals segment is expected to grow at the fastest CAGR during the forecast period.
Based on the application, the global extreme lateral interbody fusion (XLIF) surgery market is categorized as hospitals, ambulatory surgical centers, and orthopedic clinics. Among these, the hospitals segment is expected to grow at the fastest CAGR during the forecast period. Hospitals continue to play a vital role in treating the high volume of patients in need of sophisticated spinal procedures. Doctors, medical staff, and diagnostic equipment needed to perform any type of operation are present in the majority of large hospitals.
Regional Segment Analysis of the Global Extreme lateral interbody fusion (XLIF) surgery Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
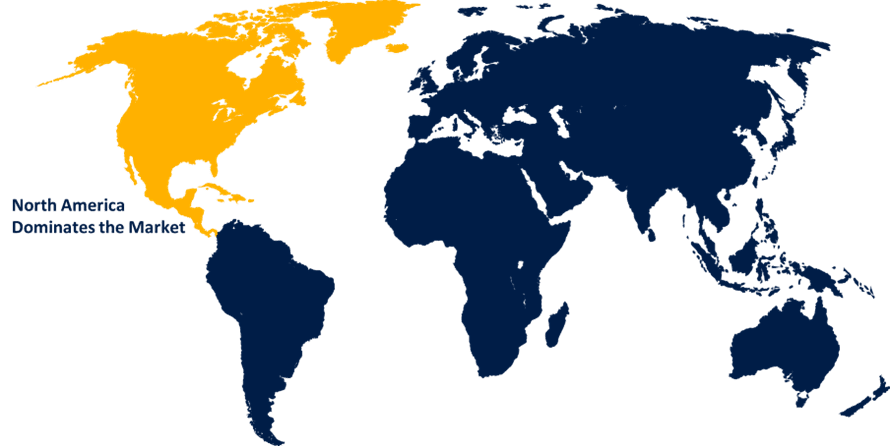
North America is projected to hold the largest share of the global extreme lateral interbody fusion (XLIF) surgery market over the forecast period.
Get more details on this report -
North America is projected to hold the largest share of the global extreme lateral interbody fusion (XLIF) surgery market over the forecast period. This is because of the region's high incidence and prevalence of spine problems. The market's growth is further supported by rising knowledge of sophisticated spinal surgery procedures and bettering healthcare infrastructure. Furthermore, the region is positioned as a prospective market due to the increased need for minimally invasive surgical procedures and rising healthcare expenditures. The US receives between 100,000 and 200,000 medical visits every year, according to the US International Trade Commission.
Europe is expected to grow at the fastest CAGR growth of the global extreme lateral interbody fusion (XLIF) surgery market during the forecast period. Europe is another important market for XLIF procedures, with nations like Germany, the UK, France, and Italy demonstrating advanced healthcare systems and rising rates of spinal disorders. An estimated 11,000 new cases of spinal-related disorders are added to the current list of people with these problems each year, according to the Council of Europe.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global extreme lateral interbody fusion (XLIF) surgery market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Medtronic plc
- Johnson & Johnson
- Stryker Corporation
- Zimmer Biomet Holdings Inc.
- NuVasive Inc.
- Globus Medical Inc.
- DePuy Synthes
- B. Braun Melsungen AG
- Alphatec Holdings Inc.
- RTI Surgical Holdings Inc.
- K2M Group Holdings Inc.
- Orthofix Medical Inc.
- Aesculap Implant Systems
- SpineWave Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global extreme lateral interbody fusion (XLIF) surgery market based on the below-mentioned segments:
Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market, By Product Type
- XLIF Interbody Fusion Systems
- XLIF Interbody Cages
Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market, By Application
- Hospitals
- Ambulatory Surgical Centers
- Orthopedic Clinics
Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the global extreme lateral interbody fusion (XLIF) surgery market over the forecast period?The global extreme lateral interbody fusion (XLIF) surgery market size is expected to grow from USD 1.93 billion in 2023 to USD 3.33 billion by 2033, at a CAGR of 5.61% during the forecast period 2023-2033.
-
2. Which region is expected to hold the highest share of the global extreme lateral interbody fusion (XLIF) surgery market?North America is projected to hold the largest share of the global extreme lateral interbody fusion (XLIF) surgery market over the forecast period.
-
3. Who are the top key players in the global extreme lateral interbody fusion (XLIF) surgery market?Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet Holdings Inc., NuVasive Inc., Globus Medical Inc., DePuy Synthes, B. Braun Melsungen AG, Alphatec Holdings Inc., RTI Surgical Holdings Inc., K2M Group Holdings Inc., Orthofix Medical Inc., Aesculap Implant Systems, SpineWave Inc., and others.
Need help to buy this report?