Global Fecal Calprotectin Test Market Size, Share, and COVID-19 Impact Analysis, By Assay Type (ELISA (Enzyme-Linked Immunosorbent Assay), Enzyme Fluroimmunoassay, and Immune-Chromatography), By Indication (Inflammatory Bowel Disease (IBD) Diagnosis, Colorectal Cancer, Celiac disease, and Others), By End-User (Hospitals, Diagnostic Laboratories, Academic and Research Institutes, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Fecal Calprotectin Test Market Insights Forecasts to 2033
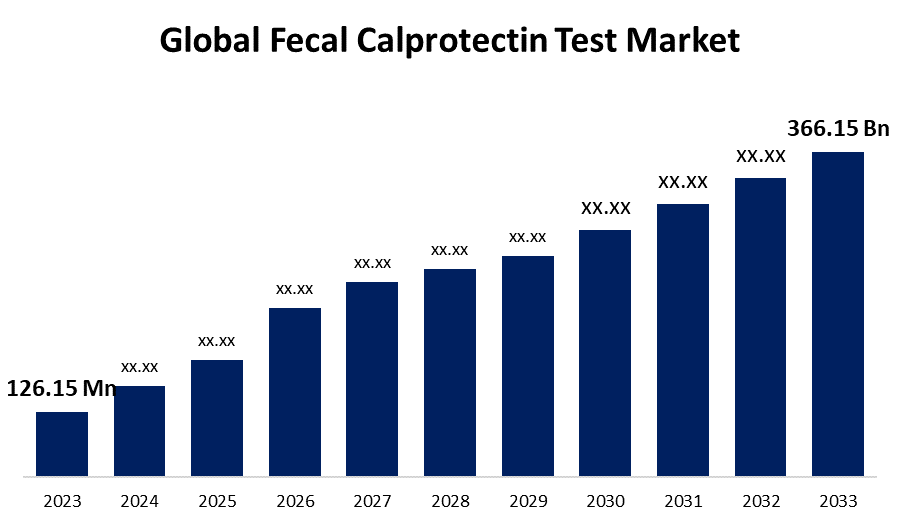
- The Global Fecal Calprotectin Test Market Size was Valued at USD 126.15 Million in 2023
- The Market Size is Growing at a CAGR of 11.24% from 2023 to 2033
- The Worldwide Fecal Calprotectin Test Market Size is Expected to Reach USD 366.15 Million by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Fecal Calprotectin Test Market Size is Anticipated to Exceed USD 366.15 Million by 2033, Growing at a CAGR of 11.24% from 2023 to 2033.
Market Overview
A calprotectin fecal test quantifies the amount of the protein calprotectin in a stool (poop) sample. Another name for the test is the fecal calprotectin test. It is used to examine intestines for inflammation, swelling, and irritation. Calprotectin is a protein found in white blood cells and is generally used as a biomarker for inflammation in the intestines. The raised level of calprotectin in stool can show the presence of inflammation in the gastrointestinal tract. To detect inflammatory bowel diseases (IBD), like Crohn’s disease and ulcerative colitis, a fecal calprotectin test is used. It is also used to alter IBD from other diseases that cause close symptoms, like irritable bowel syndrome (IBS). This test is non-invasive and can be carried out easily in a physician’s office or at home with a test kit. The patient gathers a small amount of sample of their stool and sends it to a laboratory for further analysis. The test kit is invented to provide a fast and precise result. The global market for fecal calprotectin tests is experiencing vigorous growth propelled by the rising cases of IBD and the demand for early, accurate diagnosis. In addition, a gush in research and development activities increases product portfolio, maintaining industry. Furthermore, the fulfillment in unhealthy lifestyles and consumption of junk food items has driven the development of complex gastrointestinal diseases around the world which is a positive sign for a high utilization rate of fecal calprotectin tests over the forecast period.
Report Coverage
This research report categorizes the market for the global fecal calprotectin test market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global fecal calprotectin test market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global fecal calprotectin test market.
Global Fecal Calprotectin Test Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023 : | USD 126.15 Million |
| Forecast Period: | 2023 – 2033 |
| Forecast Period CAGR 2023 – 2033 : | 11.24% |
| 023 – 2033 Value Projection: | USD 366.15 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 255 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Assay Type, By Indication, By End-User, By Region |
| Companies covered:: | Bio-Rad Laboratories, Inc., EagleBio, Svar Life Science, DRG International, Inc., ALPCO, Thermo Fisher Scientific Inc., Alpha Laboratories Ltd., Meridian Bioscience, Inc., BÜHLMANN, DIAZYME LABORATORIES, INC., Abbexa, OPERON, S.A., DiAgam, R-Biopharm AG, Biomerica, and Others Key Vendors |
| Pitfalls & Challenges: | Covid-19 Impact, Challenge, Future,Growth and Analysis |
Get more details on this report -
Driving Factors
Gastrointestinal disorders, including inflammatory bowel disease (IBD), Crohn’s disease, and ulcerative colitis are becoming more common globally. These conditions often entail chronic inflammation in the gastrointestinal tract, making the fecal calprotectin test a precious method for diagnosis and observing disease activity. Moreover, the fecal calprotectin test provides a non-invasive substitute for invasive procedures, such as colonoscopies or endoscopies. It includes collecting a stool sample, which is easier and less uncomfortable for patients. The simplicity of testing stimulates more individuals to undergo screening and monitoring, fueling the growth of the market. Additionally, healthcare workers, such as gastroenterologists and primary care physicians, are increasingly admitting the clinical utility of fecal calprotectin tests. They incorporate these tests into their diagnostic algorithms and treatment schemes, leading to a larger demand for the tests.
Restraining Factors
In a few healthcare systems, the compensation coverage for fecal calprotectin test may be restricted or inadequate. This can hamper the worldwide acquisition of these tests, as healthcare providers and patients may face economic hurdles. The absence of sufficient compensation policies and inconsistent coverage can hinder market growth. In addition, the outcomes of the fecal calprotectin test show variability due to factors, like sample collection and handling, laboratory techniques, and inter-laboratory differences. These differences can increase concerns regarding the reliability and consistency of the results of the test. Furthermore, while awareness of fecal calprotectin tests is rising, there may still be restricted awareness among healthcare professionals, especially in specific regions or medical specialties. Doctors who are not familiar with the test or its analysis may be doubtful to order or rely on fecal calprotectin tests in their clinical practice. These factors can restrain the growth of the fecal calprotectin market.
Market Segmentation
The global fecal calprotectin test market share is classified into assay type, indication, and end-user.
- The ELISA (enzyme-linked immunosorbent assay) segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period.
On the basis of the assay type, the global fecal calprotectin test market is divided into ELISA (enzyme-linked immunosorbent assay), enzyme fluroimmunoassay, and immune-chromatography. Among these, the ELISA (enzyme-linked immunosorbent assay) segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period. The ELISA test for fecal calprotectin is more precise and can identify levels as low as 15-20 µg/g of stool. This test is a non-invasive diagnostic tool that is affordable and less time-consuming than more invasive diagnostic processes, like endoscopy and colonoscopy. The ELISA test is specifically useful for the diagnosis and checking of inflammatory bowel disease (IBD) and non-IBD conditions with close symptoms, including irritable bowel syndrome (IBS).
- The inflammatory bowel disease (IBD) diagnosis segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period.
On the basis of the indication, the global fecal calprotectin test market is divided into inflammatory bowel disease (IBD) diagnosis, colorectal cancer, celiac disease, and others. Among these, the inflammatory bowel disease (IBD) diagnosis segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period. The fecal calprotectin test is first used in the diagnosis and observation of inflammatory bowel syndrome (IBD), which generally affects adults. IBD, involving Crohn’s disease and ulcerative colitis, is a chronic condition that causes inflammation in the gut, causing symptoms, like diarrhea, abdominal pain, and rectal bleeding. As a result, the cases of IBD and the rising prominence of early and precise diagnoses have driven market growth.
- The hospitals segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period.
On the basis of the end-user, the global fecal calprotectin test market is divided into hospitals, diagnostic laboratories, academic and research institutes, and others. Among these, the hospitals segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period. Hospitals are the main end-users of fecal calprotectin test, which are used for the diagnosis and observation of patients with inflammatory bowel diseases (IBD), like ulcerative colitis and Crohn’s disease. The presence of large, specialized hospitals equipped with advanced healthcare facilities and skilled professionals for consulting improves patient preference for hospitals. Accessibility of a huge range of services in hospital areas is likely to drive the segment during the forecast period.
Regional Segment Analysis of the Global Fecal Calprotectin Test Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
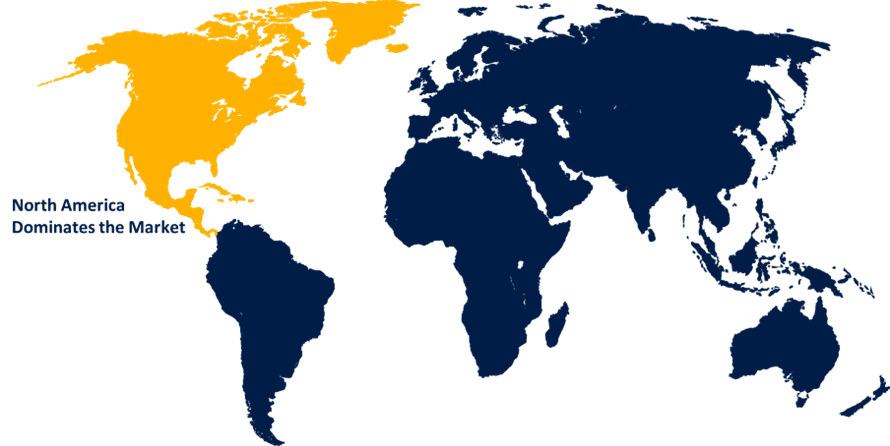
North America is anticipated to hold the largest share of the global fecal calprotectin test market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global fecal calprotectin test market over the predicted timeframe. North America has well-developed healthcare facilities and high awareness regarding gastrointestinal diseases, which led to raised acquisition of advanced diagnostic tests, such as fecal calprotectin test. In addition, the cases of inflammatory bowel disease (IBD), like ulcerative colitis and Crohn’s disease are relatively high in North America, further propelling the demand for this test. One of the major driving factors in North America is the increasing patients of with IBD. Furthermore, healthcare manufacturers are establishing advanced testing equipment to observe patients’ diseases and improve the performance of checkups. These manufacturers provide a variety of testing tools to observe the diagnostic conditions of patients and offer better service growing the United States calprotectin test market. These factors expand the fecal calprotectin test market over the predicted timeframe.
Asia-Pacific is anticipated to grow at the fastest rate in the global fecal calprotectin test market over the predicted timeframe. This region is experiencing an increasing burden of gastrointestinal diseases, such as IBD, which is propelling the demand for precise diagnostic techniques like the fecal calprotectin test. Changing lifestyle patterns, rising urbanization, and unhealthy food are predicted to contributing factors to the growing cases of gastrointestinal disorders in the Asia Pacific region. In addition, the rising awareness regarding the benefits of early disease detection and the accessibility of advanced healthcare infrastructure in nations, such as Japan, China, and South Korea are driving the adoption of fecal calprotectin test.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global fecal calprotectin test market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Bio-Rad Laboratories, Inc.
- EagleBio
- Svar Life Science
- DRG International, Inc.
- ALPCO
- Thermo Fisher Scientific Inc.
- Alpha Laboratories Ltd.
- Meridian Bioscience, Inc.
- BÜHLMANN
- DIAZYME LABORATORIES, INC.
- Abbexa
- OPERON, S.A.
- DiAgam
- R-Biopharm AG
- Biomerica
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In July 2023, Thermo Fisher Scientific Inc. completed the procure of CorEvitas, LLC (CorEvitas), a leading supplier of high-quality, real-world verification for authorized medical treatments and therapies, from Audax Private Equity (“Audax”), for a cash amount of $912.5 million. Thermo Fisher committed the deal to adopt CorEvitas.
- In September 2022, Epitope Diagnostics established a Stool Sample Quantitative Collection and Extraction Device (Sqed, US Patent pending). This sQED permits the easy and precise collection and extraction of stool specimens without the requirements for the traditional weighing method. This device also standardizes the collection method, and the extracted sample can be directly used in an automated immunoassay technique.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global fecal calprotectin test market based on the below-mentioned segments:
Global Fecal Calprotectin Test Market, By Assay Type
- ELISA (Enzyme-Linked Immunosorbent Assay)
- Enzyme Fluroimmunoassay
- Immune-Chromatography
Global Fecal Calprotectin Test Market, By Indication
- Inflammatory Bowel Disease (IBD) Diagnosis
- Colorectal Cancer
- Celiac disease
- Others
Global Fecal Calprotectin Test Market, By End-User
- Hospitals
- Diagnostic Laboratories
- Academic and Research Institutes
- Others
Global Fecal Calprotectin Test Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. Which region grows at the fastest rate in the global fecal calprotectin test market?Asia-Pacific is anticipated to grow at the fastest rate in the global fecal calprotectin test market over the predicted timeframe.
-
2. Which is the leading indication in the global fecal calprotectin test market?The inflammatory bowel disease (IBD) diagnosis segment is anticipated to hold the largest share of the global fecal calprotectin test market during the forecast period.
-
3. Which are the key players in the global fecal calprotectin test market?The key players in the global fecal calprotectin test market are Bio-Rad Laboratories, Inc., EagleBio, Svar Life Science, DRG International, Inc., ALPCO, Thermo Fisher Scientific Inc., Alpha Laboratories Ltd., Meridian Bioscience, Inc., BÜHLMANN, DIAZYME LABORATORIES, INC., Abbexa, OPERON, S.A., DiAgam, R-Biopharm AG, Biomerica, and Others.
Need help to buy this report?