Germany Biosimilars Market Size, Share, and COVID-19 Impact Analysis, By Drug Class (Monoclonal Antibodies, Insulin, Granulocyte Colony-Stimulating Factor, Erythropoietin, Recombinant Human Growth Hormone, Etanercept, Follitropin, Anticoagulants, and Other), By Indication (Oncology, Inflammatory & Autoimmune Disorders, Chronic Diseases, Blood Disorders, Growth Hormone Deficiency, Infectious Diseases, and Other), and Germany Biosimilars Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareGermany Biosimilars Market Insights Forecasts to 2033
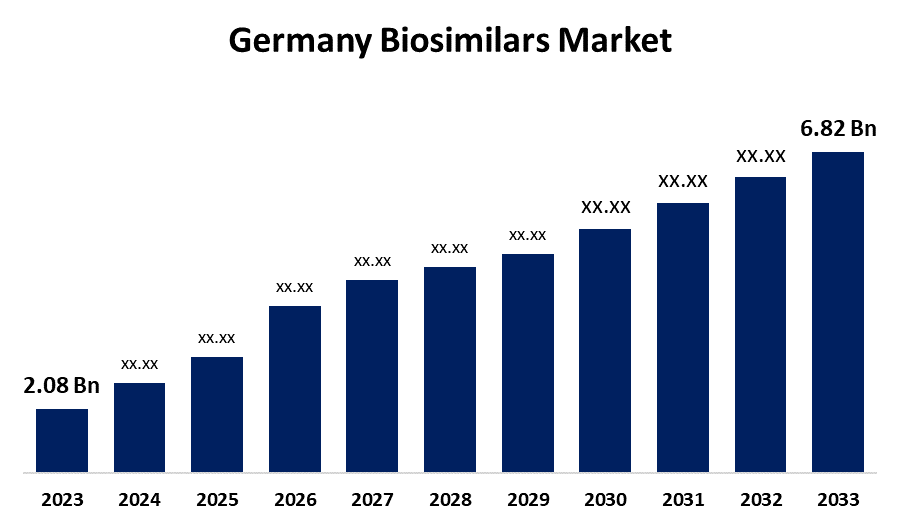
- The Germany Biosimilars Market Size was valued at USD 2.08 Billion in 2023.
- The Market Size is Growing at a CAGR of 12.61% from 2023 to 2033
- The Germany Biosimilars Market Size is Expected to Reach USD 6.82 Billion by 2033
Get more details on this report -
The Germany Biosimilars Market Size is Anticipated to Reach USD 6.82 Billion by 2033, Growing at a CAGR of 12.61% from 2023 to 2033
Market Overview
A biosimilar is a biological medicine that is highly similar to an already approved biological medication. These biosimilars closely resemble the reference medicine and demonstrate no clinically meaningful differences in safety or efficacy. They are approved using the same rigorous standards as other biological medicines. Germany leads Europe in biosimilar usage, with an uptake of approximately 50% by volume. The Drug Commission of the German Medical Association has developed guidelines for the therapeutic use of these biosimilars. In Germany, the prices of biosimilars are significantly lower than in other developed countries, and the nation has successfully fostered acceptance among payers, providers, and patients. Pharmacies can substitute a reference medication with its biosimilar or between different biosimilars. However, there are exceptions, this substitution may not occur if a physician specifically rules it out or if the pharmacy considers individual patient factors. Germany aims to further increase the uptake of biosimilars, and its companies are at the forefront of this field due to strong traditions in pharmaceuticals and chemical engineering.
Report Coverage
This research report categorizes the market for the Germany biosimilars based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Germany biosimilars market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Germany biosimilars market.
Germany Biosimilars Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 2.08 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 12.61% |
| 2033 Value Projection: | USD 6.82 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 172 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Drug Class, By Indication |
| Companies covered:: | Sandoz Group AG ADR, Biocon, Samsung Bioepis, Celltrion Healthcare, Viatris Inc, Roche, Dr. Reddy’s Laboratories, Pfizer Inc and Others |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
Several key factors are driving the biosimilar market in Germany. Biosimilars are authorized copies of biologic drugs and are typically 20–25% less expensive. This cost advantage arises from fewer clinical trials, the absence of marketing or post-marketing research and development costs, and advancements in genetic engineering. The expiration of patents for high-revenue biologic drugs is a significant catalyst for the biosimilar industry in Germany. Additionally, rising healthcare costs are encouraging patients to choose biosimilars. Government organizations are also implementing policies to incentivize the prescription of these alternatives.
Restraining Factors
Lack of awareness and high treatment costs are significant barriers to market growth. In Germany, many patients struggle to afford necessary medical care due to these factors.
Market Segmentation
The Germany biosimilars market share is classified into drug class and indication.
- The monoclonal antibodies segment is expected to hold the largest market share through the forecast period.
The Germany biosimilars market is segmented by drug class into monoclonal antibodies, insulin, granulocyte colony-stimulating factor, erythropoietin, recombinant human growth hormone, etanercept, follitropin, anticoagulants, and others. Among these, the monoclonal antibodies segment is expected to hold the largest market share through the forecast period. Monoclonal antibodies are widely utilized in the management of conditions like cancer, rheumatoid arthritis, cardiovascular diseases, and multiple sclerosis. These antibodies specifically target infected cells during treatment, making them particularly valuable in cancer therapy.
- The oncology segment is expected to dominate the Germany biosimilars market during the forecast period.
Based on the indication, the Germany biosimilars market is divided into oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, growth hormone deficiency, infectious diseases, and others. Among these, the oncology segment is expected to dominate the Germany biosimilars market during the forecast period. Primarily due to the availability of biosimilars at lower prices compared to innovative biologics, as well as the significant number of cancer patients. Introducing biosimilars in oncology has helped reduce costs, making cancer treatment more affordable and accessible. Additionally, given the high incidence and prevalence of cancer, healthcare systems worldwide are focusing on alleviating the burden of the disease by adopting cost-effective treatment options.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Germany biosimilars market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sandoz Group AG ADR
- Biocon
- Samsung Bioepis
- Celltrion Healthcare
- Viatris Inc
- Roche
- Dr. Reddy’s Laboratories
- Pfizer Inc
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In January 2024, Sandoz, a global leader in generic and biosimilar medicines, announces the launch of Tyruko (natalizumab) in Germany starting February 1. Developed by Polpharma Biologics, Tyruko is the first and only biosimilar approved for treating RRMS.
Market Segment
This study forecasts revenue at Germany, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Germany Biosimilars Market based on the below-mentioned segments:
Germany Biosimilars Market, By Drug Class
- Monoclonal Antibodies
- Insulin
- Granulocyte Colony-Stimulating Factor
- Erythropoietin
- Recombinant Human Growth Hormone
- Etanercept
- Follitropin
- Anticoagulants
- Other
Germany Biosimilars Market, By Indication
- Oncology
- Inflammatory & Autoimmune Disorders
- Chronic Diseases
- Blood Disorders
- Growth Hormone Deficiency
- Infectious Diseases
- Other
Need help to buy this report?