Germany Epilepsy Devices Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Conventional and Wearable Devices, EEG, Electrocardiography (EKG), Surface Electromyography (sEMG), Video Detection Systems, and Others), By Technology (Deep Brain Stimulation, Vagus Nerve Stimulation, Responsive Neurostimulation, and Accelerometry), and Germany Epilepsy Devices Market Insights, Industry Trend, Forecasts to 2033.
Industry: HealthcareGermany Epilepsy Devices Market Insights Forecasts to 2033
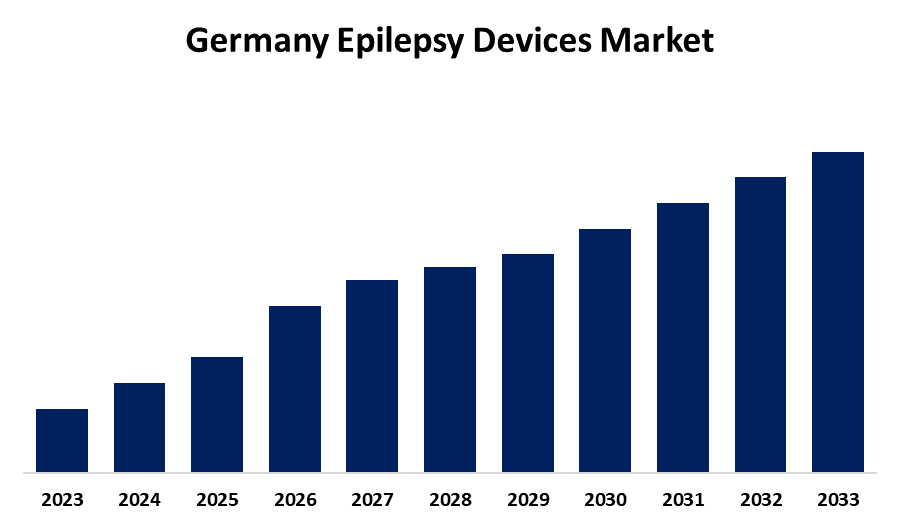
- The Germany Epilepsy Devices Market is Growing at a CAGR of 5.14% from 2023 to 2033
- The Germany Epilepsy Devices Market Size is Expected to Reach a Significant Share by 2033
Get more details on this report -
The Germany Epilepsy Devices Market is anticipated to reach a significant share by 2033, growing at a CAGR of 5.14% from 2023 to 2033.
Market Overview
Germany epilepsy devices market includes products used to diagnose, monitor, and treat epilepsy. These devices, such as brain stimulation systems and seizure detection wearables, help manage epilepsy more effectively. The Germany epilepsy devices market is experiencing steady growth due to advancements in medical technology, increased awareness of epilepsy, and a rising number of epilepsy cases. These factors are encouraging the demand for devices that can accurately diagnose, monitor, and treat epilepsy, such as neurostimulation systems and seizure detection wearables. Opportunities in the market lie in the development of more personalized and non-invasive treatment options, as well as the integration of AI and data analytics for better seizure prediction and management. Additionally, the German government has been actively supporting epilepsy treatment through healthcare reforms and funding for medical research, creating a favorable environment for innovation in epilepsy care. These government initiatives, along with collaborations between healthcare providers and technology companies, are expected to further drive the market's expansion.
Report Coverage
This research report categorizes the market for the Germany epilepsy devices market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Germany epilepsy devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Germany epilepsy devices market.
Germany Epilepsy Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 5.14% |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 111 |
| Segments covered: | By Product Type, By Technology. |
| Companies covered:: | Cadwell Industries, Abbott Laboratories, Natus Medical Incorporated, Medtronic plc, Empatica, Inc, Compumedics Limited, Medpage Ltd, Boston Scientific Corporation, Masimo Corporation, LivaNova PLC, Others, |
| Pitfalls & Challenges: | COVID-19 Empact,Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Technological innovations such as improved neurostimulation devices, wearable seizure detection systems, and enhanced diagnostic tools are making epilepsy management more effective. Rising awareness about the impact of epilepsy on individuals and the healthcare system is leading to greater demand for advanced treatment options. Additionally, the growing number of epilepsy cases in Germany, coupled with an aging population, is further driving the need for better diagnostic and therapeutic devices to manage the condition more efficiently.
Restraining Factors
Lack of sufficient awareness and training among healthcare providers regarding the latest epilepsy devices may hinder effective implementation, slowing market growth.
Market Segmentation
The Germany epilepsy devices market share is classified into product type and technology.
- The conventional and wearable devices segment accounted for the major revenue share in 2023 and is expected to grow at a significant CAGR during the forecast period.
The Germany epilepsy devices market is segmented by product type into conventional and wearable devices, EEG, electrocardiography (EKG), surface electromyography (sEMG). Among these, the conventional and wearable devices segment accounted for the major revenue share in 2023 and is expected to grow at a significant CAGR during the forecast period. The growth is driven by the increasing demand for user-friendly, non-invasive devices that can be easily worn by patients for continuous monitoring and seizure detection, improving overall patient care and convenience.
- The deep brain stimulation segment accounted for the largest revenue share in 2023 and is expected to grow at a substantial CAGR during the forecast period.
The Germany epilepsy devices market is segmented by technology into deep brain stimulation, vagus nerve stimulation, responsive neurostimulation, and accelerometry. Among these, the deep brain stimulation segment accounted for the largest revenue share in 2023 and is expected to grow at a substantial CAGR during the forecast period. The segment growth is attributed to the effectiveness of DBS in managing drug-resistant epilepsy by targeting specific brain areas to reduce seizures, as well as increasing adoption of advanced neurostimulation therapies for better patient outcomes.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Germany epilepsy devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Cadwell Industries
- Abbott Laboratories
- Natus Medical Incorporated
- Medtronic plc
- Empatica, Inc
- Compumedics Limited
- Medpage Ltd
- Boston Scientific Corporation
- Masimo Corporation
- LivaNova PLC
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In February 2024, Empatica launched the EpiMonitor, a next-generation wearable epilepsy monitoring system. This device, FDA-cleared for adults and children aged six and above, features automatic seizure detection with a 98% accuracy rate, a week-long battery life, and advanced health insights. It also includes a companion app for seizure tracking and caregiver alerts.
Market Segment
This study forecasts revenue at Germany, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Germany epilepsy devices market based on the below-mentioned segments:
Germany Epilepsy Devices Market, By Product Type
- Conventional and Wearable Devices
- EEG
- Electrocardiography (EKG)
- Surface Electromyography (sEMG)
- Video Detection Systems
- Others
Germany Epilepsy Devices Market, By Technology
- Deep Brain Stimulation
- Vagus Nerve Stimulation
- Responsive Neurostimulation
- Accelerometry
Need help to buy this report?