Global GMP Biologics Market Size, Share, and COVID-19 Impact Analysis, By Type (Monoclonal Antibodies and Polyclonal Antibodies), By Application (Hospitals, Clinics, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal GMP Biologics Market Insights Forecasts to 2033
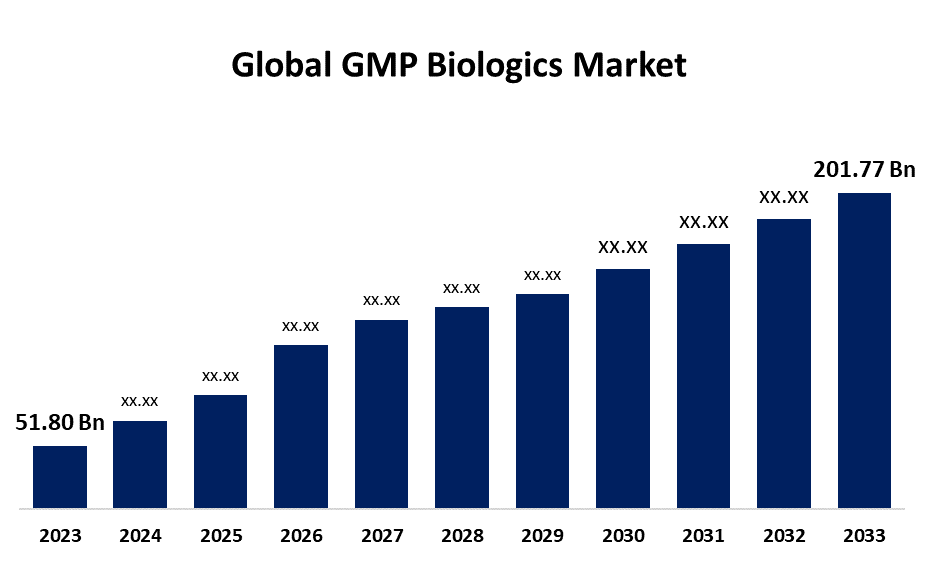
- The Global GMP Biologics Market Size was Valued at USD 51.80 Billion in 2023
- The Market Size is Growing at a CAGR of 14.57% from 2023 to 2033
- The Worldwide GMP Biologics Market Size is Expected to Reach USD 201.77 Billion by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global GMP Biologics Market Size is Anticipated to Exceed USD 201.77 Billion by 2033, Growing at a CAGR of 14.57% from 2023 to 2033.
Market Overview
A collection of rules and regulations known as good manufacturing practices (GMP) for biologics is designed to guarantee the uniform production of biological products, including vaccines while assuring their safety and quality. Moreover, to ensure customer safety, GMP supports preserving the final product's integrity and quality. GMP biologics standards, set by the World Health Organization (WHO) and other organizations, mandate that biologics manufacturing procedures be monitored and documented to reduce risks such as contamination and ensure consistency between production batches. For Instance, in October 2024, to produce monoclonal antibodies (mAbs) and protein treatments for clinical and commercial uses, Eurofins CDMO Alphora Inc. announced motives to build a new GMP Biologics manufacturing facility in Mississauga, Ontario. The growing usage of monoclonal antibodies and biosimilars, technological advancements in bioprocessing, and a growing focus on tailored care are the main factors driving the GMP biologics market.
Report Coverage
This research report categorizes the GMP biologics market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the GMP biologics market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the GMP biologics market.
Global GMP Biologics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 51.80 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 14.57% |
| 2033 Value Projection: | USD 51.80 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 264 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Type, By Application, By Region |
| Companies covered:: | Amgen Inc., Thermo Fisher Scientific, Creative Diagnostics, Hoffmann-La Roche Ltd, AstraZeneca plc, WuXi Biologics, Merck KGaA, AbbVie Inc., HemaCare, AstraZeneca plc, Novartis AG, Polpharma Biologics, Creative Diagnostics, Intertek, and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The rising need for biopharmaceuticals, which include medicinal proteins, vaccines, and monoclonal antibodies, is one of the main factors driving the GMP biologics market. Due to biologics offering specialized and effective treatments for a variety of illnesses, there is a growing need for biologics manufacturing that complies with GMP standards. The need for the GMP biologics market is being driven by the rising prevalence of chronic diseases such as cancer, autoimmune diseases, and infectious diseases. The rising biopharmaceutical demand and growing biologics pipeline are the main factors driving the growth in the GMP biologics market.
Restraining Factors
The intricacy of these processes can lead to issues with scalability, efficiency, and process optimization that can restrict the overall cost-effectiveness production of the GMP biologics market.
Market Segmentation
The GMP biologics market share is classified into type and application.
- The monoclonal antibodies segment is estimated to hold the largest market revenue share through the projected period.
Based on the type, the GMP biologics market is classified into monoclonal antibodies and polyclonal antibodies. Among these, the monoclonal antibodies segment is estimated to hold the largest market revenue share through the projected period. The monoclonal antibodies (MAbs) can specifically target certain antigens, they are widely used in clinical and hospital settings to improve treatment success and minimize side effects.
- The hospital segment is anticipated to hold the largest market share through the forecast period.
Based on the application, the GMP biologics market is divided into hospitals, clinics, and others. Among these, the hospital segment is anticipated to hold the largest market share through the forecast period. The hospitals serve as the main hubs for cutting-edge medical care, including gene therapies, biologics such as monoclonal antibodies, and other specialty treatments.
Regional Segment Analysis of the GMP Biologics Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
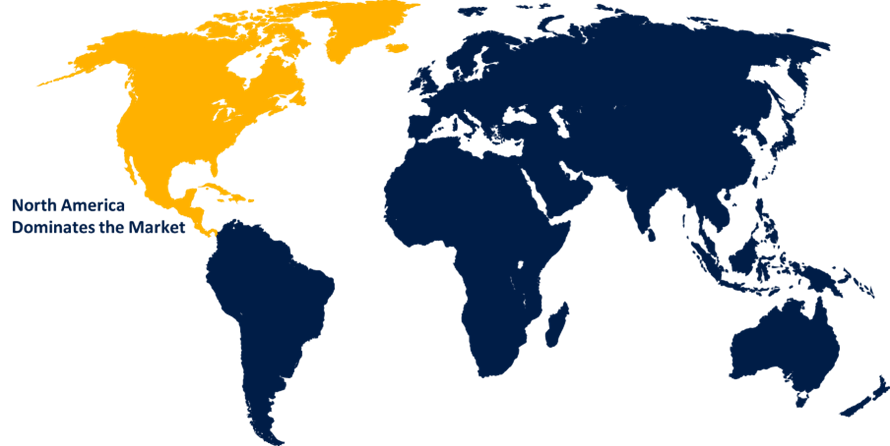
North America is anticipated to hold the largest share of the GMP biologics market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the GMP biologics market over the predicted timeframe. In North America there are numerous respectable biopharmaceutical companies and a robust regulatory framework that encourages the creation of biologic medications. The region has a strong infrastructure that is necessary for the manufacture and delivery of biologics, including top manufacturing facilities, supply networks, and research institutes. Strong regulatory policies that assist the approval and commercialization of biological drugs also contribute to the growth of this region.
Asia Pacific is expected to grow at the fastest CAGR growth of the GMP biologics market during the forecast period. The Asia-Pacific region has seen a notable surge in biopharmaceutical-related research and development efforts. Governments, academic institutions, and commercial companies are investing in the development of novel biologics for a variety of therapeutic purposes, which is driving the need for GMP-compliant production.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the GMP biologics market along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Amgen Inc.
- Thermo Fisher Scientific
- Creative Diagnostics
- Hoffmann-La Roche Ltd
- AstraZeneca plc
- WuXi Biologics
- Merck KGaA
- AbbVie Inc.
- HemaCare
- AstraZeneca plc
- Novartis AG
- Polpharma Biologics
- Creative Diagnostics
- Intertek
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In August 2024, the world-renowned Contract Research, Development and Manufacturing Organization (CRDMO), WuXi Biologics ("WuXi Bio") (2269. HK), announced that it has effectively completed 2,000L drug substance (DS) GMP manufacturing through the use of its exclusive ultra-intensified fed-batch bioprocessing platform WuXiUITM.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the GMP biologics market based on the below-mentioned segments:
Global GMP Biologics Market, By Type
- Monoclonal Antibodies
- Polyclonal Antibody
Global GMP Biologics Market, By Application
- Hospitals
- Clinics
- Others
Global GMP Biologics Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the GMP biologics market over the forecast period?The GMP biologics market is projected to expand at a CAGR of 14.57% during the forecast period.
-
2. What is the market size of the GMP biologics market?The Global GMP Biologics Market Size is Expected to Grow from USD 51.80 Billion in 2023 to USD 201.77 Billion by 2033, at a CAGR of 14.57% during the forecast period 2023-2033.
-
3. Which region holds the largest share of the GMP biologics market?North America is anticipated to hold the largest share of the GMP biologics market over the predicted timeframe.
Need help to buy this report?