Global Hernia Mesh Devices Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Synthetic Mesh, Biologic Mesh, and Composite Mesh), By Hernia Type (Inguinal, Incisional, Femoral, Umbilical and Other Hernias), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033.
Industry: HealthcareGlobal Hernia Mesh Devices Market Insights Forecasts to 2033
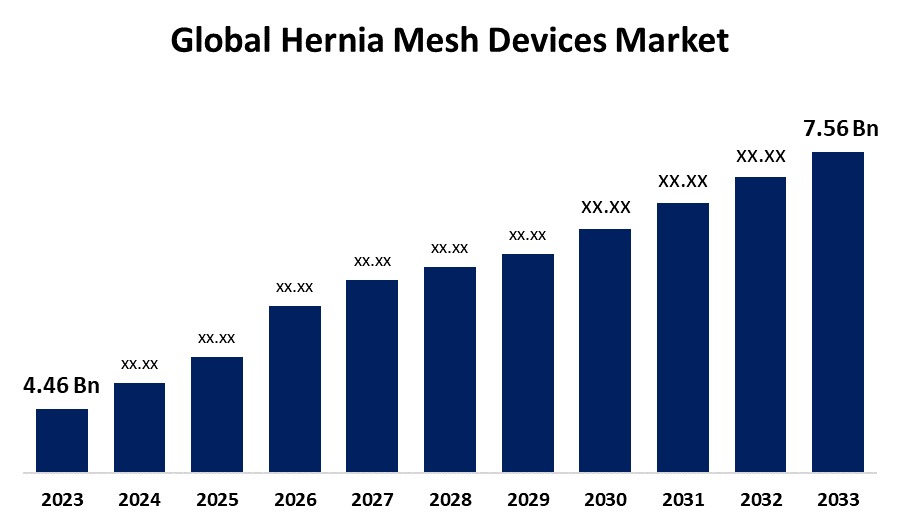
- The Global Hernia Mesh Devices Market Size was Valued at USD 4.46 Billion in 2023
- The Market Size is Growing at a CAGR of 5.42% from 2023 to 2033
- The Worldwide Hernia Mesh Devices Market Size is Expected to Reach USD 7.56 Billion by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Hernia Mesh Devices Market Size is Anticipated to Exceed USD 7.56 Billion by 2033, Growing at a CAGR of 5.42% from 2023 to 2033.
Market Overview
A medical device called hernia mesh helps in the repair of injured tissue around hernias. It is made of both absorbable and non-absorbable materials, and in order to allow tissues to grow into the device, ligaments, staples, or glue are needed. To lower the risk of hernia recurrence and strengthen the hernia repair, it is inserted into the patient's upper stomach, groin, or abdomen during hernia surgery. Additionally, by cutting down on recovery time and operating time, it enhances patient outcomes. Consequently, ambulatory surgery facilities, clinics, and hospitals use hernia mesh devices extensively. Hernia mesh devices are intended to strengthen and reinforce the weaker tissue, speeding up the healing process and lowering the chance of recurrence. They are usually constructed of synthetic materials like polypropylene or bioabsorbable materials. Innovations like articulating fixation and self-fixating meshes are expected to resolve the problems, which is a major reason for the rise in the use of hernia mesh devices. A hernia mesh is a permanent implant that should last a lifetime, according to the FDA. A number including smoking, poor nutrition, genetics, obesity, and changes in lifestyle, are linked to the higher-than-average prevalence of hernias.
Report Coverage
This research report categorizes the market for the global hernia mesh devices market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global hernia mesh devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global hernia mesh devices market.
Global Hernia Mesh Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 4.46 Billion |
| Forecast Period: | 2023 to 2033 |
| Forecast Period CAGR 2023 to 2033 : | 5.42% |
| 2033 Value Projection: | USD 7.56 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 231 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product Type, By Hernia Type, By Region and COVID-19 Impact Analysis |
| Companies covered:: | Johnson & Johnson Services, Inc., C. R. Bard, Inc., W. L. Gore & Associates, Inc., Atrium Medical (Getinge Group), LifeCell, B. Braun SE, Baxter, Cook, Herniamesh S.r.l., Ethicon, B. Braun Melsungen AG., PRIMEQUAL SA, Becton, Dickinson, and Company, Deep Blue Medical Inc, Dipromed Srl, BioCer Entwicklungs-GmbH, and others Key players. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Hernia mesh is a flexible medical device that supports damaged tissue around a hernia. Umbilical, inguinal, and incisional hernias are among the many types of hernia repairs that it is used to treat. A common medical condition that affects millions of people globally is hernias. Hernias are becoming increasingly widespread as a result of factors including obesity, aging populations, and sedentary lifestyles. This raises the need for hernia repair procedures and, eventually, hernia mesh systems. Technological advances in surgical procedures, including minimally invasive therapies like surgical hernia repair, have improved the safety and efficacy of hernia repair surgery. The market for these devices is expanding because of the significance of hernia mesh devices in these procedures. Mesh repair is becoming more and more used for treating hernias since it produces better results and has a lower recurrence rate than standard suture repair. This trend drives the demand for hernia mesh devices. Expanding demand for biologic meshes, biomedical meshes composed of human or animal tissue are gaining popularity due to their superior tissue integration and reduced infection risk compared to alternative materials. The better access to healthcare services together with rising healthcare spending in developed and developing countries are driving the need for hernia repair treatments like hernia mesh devices.
Restraining Factors
The complications from hernia surgery include bleeding, infection at the surgical site, bladder damage, vascular damage, and intestinal damage. Adhesion, intestinal obstruction, discomfort during migration, and persistent pain are complications linked to hernia mesh that are preventing the market's income from growing. There have been reports of hernia mesh implant complications in certain cases, including as infections, adhesion, mesh migration, and persistent discomfort. Safety issues may prevent the market from growing as users and medical professionals become more hesitant to accept these devices. Strict guidelines for hernia mesh device approval may prevent the entry of new businesses and postpone the release of innovative products. Hernia mesh devices can be too expensive for patients and healthcare systems to afford. Financial challenges may make it more difficult to utilize these devices, especially in places where healthcare resources are limited or where compensation is a challenge.
Market Segmentation
The global hernia mesh devices market share is classified into product type, hernia type.
- The synthetic Mesh is expected to hold the largest share of the global hernia mesh devices market during the forecast period.
Based on the product type, the global hernia Mesh devices market is divided into synthetic Mesh, biologic Mesh, and composite Mesh. Among these, the synthetic Mesh is expected to hold the largest share of the global hernia Mesh devices market during the forecast period. The market for hernia mesh was dominated by synthetic meshes because of their affordability, simplicity of buying of product materials, and high product availability. When it came to the sales of mesh devices, synthetic meshes dominated the market due in large part to their ease of availability, affordability, and recent product launches. Furthermore, synthetic meshes have less surgical problems than biological meshes and are more cost-effective for the majority of patients.
- The inguinal hernia is expected to hold the largest share of the global hernia mesh devices market during the forecast period.
Based on the hernia type, the global hernia Mesh devices market is divided into inguinal, incisional, femoral, umbilical, and other hernias. Among these, the inguinal hernia is expected to hold the largest share of the global hernia Mesh devices market during the forecast period. The most frequent operation in general surgery is the correction of inguinal hernias. Organs located within or outside the abdomen may emerge via a gap in the lateral and muscular plain. Most people have some sort of groin bulge or pain when they first arise. Medical professionals advise that all symptomatic hernias be treated to prevent complications.
Regional Segment Analysis of the global hernia mesh devices market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
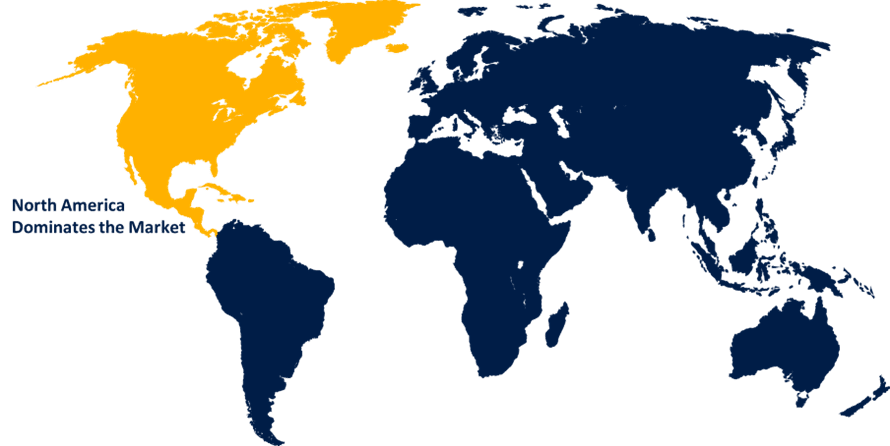
North America is anticipated to hold the largest share of the global hernia mesh devices market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global hernia Mesh devices market over the predicted timeframe. The key contributing factors include the vast number of persons affected by the illness, improved healthcare facilities, sedentary lifestyles, and the increasing use of surgical meshes as a treatment option. Government and industry factors have made precise reimbursement codes and ratios easily accessible to patients. A few of the reasons driving the market expansion in North America include a lack of exercise, an aging population, and the high prevalence of hernia recurrence. Moreover, the region's market is expanding as a result of growing healthcare costs and faster FDA approvals.
Asia Pacific is expected to grow at the fastest pace in the global hernia mesh devices market during the forecast period. A few of the reasons propelling the market in the region include growing healthcare reimbursements, medical tourism, economical treatment, and technological developments. Moreover, because of a sizable patient base, Asian nations—particularly China and Japan are experiencing a surge in demand for hernia repair equipment. Due to the high number of instances that remain misdiagnosed and untreated, the region is anticipated to have substantial expansion, which will in turn drive the growth of the market in this region.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global hernia mesh devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Johnson & Johnson Services, Inc.
- C. R. Bard, Inc.
- W. L. Gore & Associates, Inc.
- Atrium Medical (Getinge Group)
- LifeCell
- B. Braun SE
- Baxter
- Cook
- Herniamesh S.r.l.
- Ethicon
- B. Braun Melsungen AG.
- PRIMEQUAL SA
- Becton, Dickinson, and Company
- Deep Blue Medical Inc
- Dipromed Srl
- BioCer Entwicklungs-GmbH
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In April 2022 – The FDA granted Ariste Medical, a medical device firm, permission under section 501(k) to distribute drug-embedded synthetic hernia mesh in the United States.
- In July 2022 - To market its hernia repair solutions, Deep Blue Medical Advances, Inc. increased its venture financing to over USD 7.0 million.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global hernia mesh devices market based on the below-mentioned segments:
Global Hernia Mesh Devices Market, By Product Type
- Synthetic Mesh
- Biologic Mesh
- Composite Mesh
Global Hernia Mesh Device Market, By Hernia Type
- Inguinal
- Umbilical
- Incisional
- Femoral
- Others
Global Hernia Mesh Devices Market, Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. Which are the key companies that are currently operating within the market?Johnson & Johnson Services, Inc., C. R. Bard, Inc., W. L. Gore & Associates, Inc., Atrium Medical (Getinge Group), LifeCell, B. Braun SE, Baxter, Cook, Herniamesh S.r.l., Others
-
2. What is the size of the global hernia mesh devices market?The global hernia mesh devices market is expected to grow from USD 4.46 Billion in 2023 to USD 7.56 Billion by 2033, at a CAGR of 5.42% during the forecast period 2023-2033.
-
3. Which region is holding the largest share of the market?North America is anticipated to hold the largest share of the global hernia mesh devices market over the predicted timeframe.
Need help to buy this report?