Italy In Vitro Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (Instruments, Reagents, Services), By Technology, (Immunoassay, Hematology, Clinical Chemistry, Molecular Diagnostics/Genetics, Coagulation, Microbiology, and Others), By Application (Infectious Disease, Diabetes, Oncology/Cancer), By End-use, (Core Lab, Hematology, Coagulation, Urine Test, and Others), and Italy In Vitro Diagnostics Market Insights Forecasts 2023 - 2033.
Industry: HealthcareItaly In Vitro Diagnostics Market Insights Forecasts to 2033
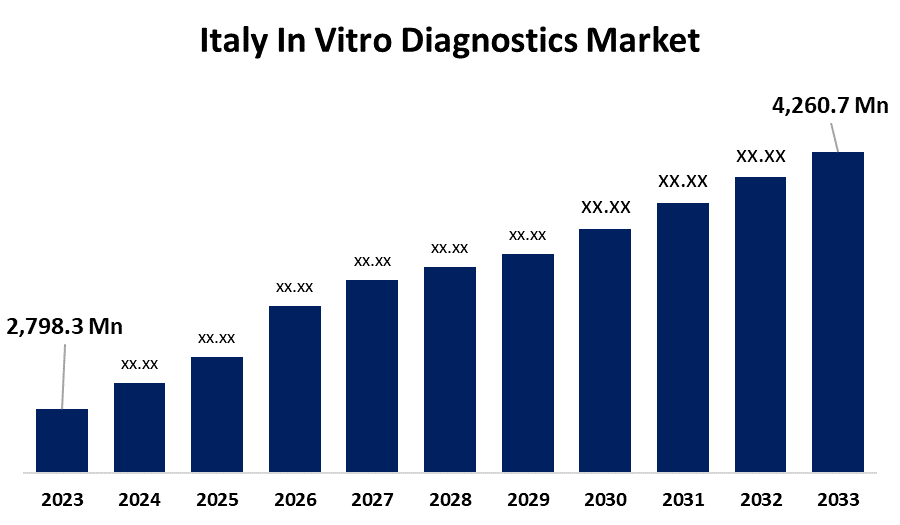
- The Italy In Vitro Diagnostics Market Size was valued At USD 2,798.3 Million in 2023
- The Market Size is Growing at 4.29% CAGR from 2023 to 2033.
- The Italy In Vitro Diagnostics Market Size is Expected to reach USD 4,260.7 Million by 2033.
Get more details on this report -
The Italy In Vitro Diagnostics Market Size is Expected to reach At USD 4,260.7 Million by 2033, Growing at a 4.29% CAGR from 2023 to 2033.
Market Overview
The Italy in vitro diagnostics test is used to identify illnesses, ailments, and infections. In contrast to in vivo examinations, which are carried out in the body itself, in vitro tests are usually carried out in test tubes and comparable apparatus. The term "in vitro" literally means "in glass." In vitro testing can be conducted at home, in a lab, or a medical facility. The tests themselves can be conducted using a range of equipment, from simple handheld testing to sophisticated lab equipment. Moreover, Italy IVD market will rise because of increased chronic disease prevalence, population aging, and an increasing need for early detection of diseases. Improved technology in molecular diagnostics, automation, and AI-based testing leads to greater efficiency and accuracy. In addition, government health care initiatives, an expansion in point-of-care testing, and a growing need for personalized medicine increase the scope for the IVD market and ensure its use as an important area of health care for future Italy. Furthermore, Italy's in vitro diagnostics market continues to grow at a rapid rate due to demand for rapid diagnostics, increased uptake of home testing, biomarker research advancements, digital health incorporation, and greater investments in AI-powered and automated laboratory solutions.
Report Coverage
This research report categorizes the market for the Italy in vitro diagnostics market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Italy in vitro diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Italy in vitro diagnostics market.
Italy In Vitro Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 2,798.3 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 4.29% |
| 2033 Value Projection: | USD 4,260.7 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 245 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By Technology, By Application, By End-use |
| Companies covered:: | bioMerieux SA, Danaher, Sysmex Corporation, QIAGEN, The Menarini Group, DiaSorin, Abbott Laboratories, and BD |
| Pitfalls & Challenges: | COVID-19 impact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Increased cases of infectious diseases, expanding infrastructure in laboratories, and increased health-care spending form the driving factors behind the Italian IVD market. Increasing usage of point-of-care testing, need for non-invasive diagnostic practices, and increased preventive care promote growth in this market. Besides, R&D investments in strong proportions, the integration of genomics-based tests, and conducive regulatory environment ensure that growth occurs at an enhanced pace to render IVD more indispensable to achieve better patient management and treatment results.
Restraining Factors
Some challenges that the IVD market of Italy faces are high costs associated with advanced diagnostic tests, rigorous regulatory approvals, reimbursement limitations, data privacy issues, and the shortage of qualified professionals, thus limiting its use and growth.
Market Segment
- The reagent segment held the largest share in 2023 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the product, the Italy in vitro diagnostics market is divided into instruments, reagents, and services. Among these, the reagent segment held the largest share in 2023 and is anticipated to grow at a significant CAGR during the forecast period. This is due to the repeated demand in diagnostic procedures, increasing adoption of molecular and immunoassay tests, and advancements in biomarker-based reagents. Its strong growth trajectory is driven by the rising prevalence of infectious and chronic diseases and continuous innovation in specialized reagents for personalized medicine and rapid testing.
- The molecular diagnostics/genetics segment accounted for the majority of the share in 2023 and is estimated to grow at a significant CAGR during the projected timeframe.
Based on the technology, the Italy In vitro diagnostics market is divided into immunoassay, hematology, clinical chemistry, molecular diagnostics/genetics, coagulation, microbiology, and others. Among these, the molecular diagnostics/genetics segment accounted for the majority of the share in 2023 and is estimated to grow at a significant CAGR during the projected timeframe. The segment's market share has grown dramatically as a consequence of the key IVD market participants' ongoing involvement with molecular diagnostics technologies, advances in molecular diagnostics, and a spike in product launches. The precision and speed of molecular diagnostics are being improved by ongoing advancements in genetic sequencing technologies, such as polymerase chain reaction (PCR) and next-generation sequencing (NGS). Because of this, it is a favoured technique for identifying cancer, infectious diseases, and genetic disorders.
- The infectious disease segment accounted for the lead market share in 2023 and is estimated to grow at a significant CAGR during the estimated period.
Based on the application, the Italy In vitro diagnostics market is classified into infectious disease, diabetes, and oncology/cancer. Among these, the infectious disease segment accounted for the lead market share in 2023 and is estimated to grow at a significant CAGR during the estimated period. The growing prevalence of infectious diseases and the growing use of IVD products for infectious disease detection are the reasons for the infectious disease segment's high IVD market share. The segment's expansion is being driven by the rising rates of HIV, TB, pneumonia, hepatitis, and other infectious disorders. With the increase in drug addiction cases, medication errors, and the need for individualized healthcare, the drug testing and oncology segment is expected to rise at a quicker rate over the forecast period. Additionally, it is anticipated that the increase in cancer incidence and growing knowledge of early illness detection will accelerate sector growth.
- The core lab segment accounted for the largest market share in 2023 and is estimated to grow at a significant CAGR during the estimated period.
Based on the end-use, the Italy In vitro diagnostics market is classified into core lab, hematology, coagulation, urine test, and others. Among these, the core lab segment accounted for the largest market share in 2023 and is estimated to grow at a significant CAGR during the estimated period. The growing number of core laboratories conducting diagnostic tests at clinics, hospitals, labs, and other healthcare facilities is responsible for the core lab segment's growth. Technological developments, the capacity to conduct a wide range of diagnostic tests, and an increase in the demand for reasonably priced services are further reasons driving the segment's growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Italy in vitro diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- bioMerieux SA
- Danaher
- Sysmex Corporation
- QIAGEN
- The Menarini Group
- DiaSorin
- Abbott Laboratories
- BD
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at regional, and country levels from 2023 to 2033. Spherical Insights has segmented the Italy in vitro diagnostics market based on the below-mentioned segments:
Italy In Vitro Diagnostics Market, By Product
- Instruments
- Reagents
- Services
Italy In Vitro Diagnostics Market, By Technology
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostics/Genetics
- Coagulation
- Microbiology
- Others
Italy In Vitro Diagnostics Market, By Application
- Infectious Disease
- Diabetes
- Oncology/Cancer
Italy In Vitro Diagnostics Market, By End-use
- Core Lab
- Hematology
- Coagulation
- Urine Test
- Others
Need help to buy this report?