Japan Cardiovascular Devices Market Size, Share, and COVID-19 Impact Analysis, By Device Type (Diagnostic & Monitoring Devices {Electrocardiogram (ECG), Remote Cardiac Monitoring, Others}, Therapeutic & Surgical Devices {Cardiac Assist Devices, Cardiac Rhythm Management Devices, Catheter, Grafts, Heart Valves, Stents, Others}), and Japan Cardiovascular Devices Market Insights, Industry Trend, Forecasts to 2030
Industry: HealthcareThe Japan Cardiovascular Devices Market Size is expected to grow measurable count at a CAGR of 4.3% during the forecast period 2022-2030. The increasing burden of cardiovascular diseases and the increased adoption of minimally invasive procedures may lead to grow the Japan Market.
Get more details on this report -
Market Overview
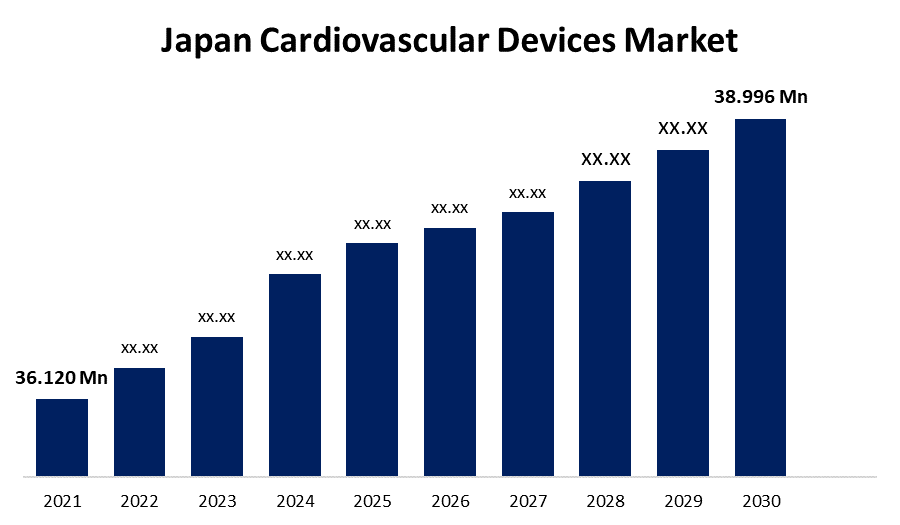
The most dependable and effective method for monitoring a variety of cardiac problems is cardiac monitoring. According to the World Ageing 2019 report, Japan has the world’s fastest-aging population, which estimates the number of populations who are above 65 years of age in Japan was 36.120 million (28%) in 2019 which is projected to reach 38.996 million (30.9%) in 2030. As the geriatric population is expected to increase in the coming years.
Report Coverage
This research report categorizes the market for Japan Cardiovascular Devices Market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan Cardiovascular Devices Market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segments of the Japan Cardiovascular Devices Market.
Japan Cardiovascular Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2021 |
| Market Size in 2021: | 36.120 Million |
| Forecast Period: | 2021-2030 |
| Forecast Period CAGR 2021-2030 : | 4.3% |
| 2030 Value Projection: | 38.996 Million |
| Historical Data for: | 2017-2020 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 130 |
| Segments covered: | By Device Type, and COVID-19 Impact Analysis |
| Companies covered:: | Abbott Laboratories, Boston Scientific Corporation, Edwards Lifesciences, Medtronic PLC, Cardinal Health Inc., Biotronik, Siemens Healthineers AG, W. L. Gore & Associates, Inc., Canon Medical Systems Corporation |
| Pitfalls & Challenges: | COVID-19 has the potential to impact the global market |
Get more details on this report -
Driving Factors
The factors include driving the market as leading market participants in cardiac monitoring devices are expected to make several advances, product approvals, launches, partnerships, and acquisitions, rise in technological advancement that would likely encourage the growth of the market throughout the forecast period. Moreover, through the studied segment, the market is expected to grow owing to the increasing aging population and the rising product launch approvals by various market players, for example, in September 2021, Astellas Pharma Inc., Nitto Denko Corporation and M. Heart Co., Ltd. entered a memorandum of understanding on concerning an ECG testing service.
Restraining Factors
Growth of the market is forecasted to be hampered by unfavorable compensate the policies and the infection risk is related to the implantable monitoring devices. Moreover, stringent regulatory policies, along with the high cost of instruments and procedures are expected to hinder the market growth.
COVID 19 Impacts
The COVID-19 outbreak has had an impact on all industries, including Japan's Cardiovascular Devices Market due to a decrease in cardiovascular surgeries during the pandemic. The decrease in procedures during the pandemic compacted the growth of the cardiovascular devices market due to less usage of these devices. Thus, the pandemic has subsided, the market is expected to increase purchase in the pandemic due to the resumption of cardiovascular surgical procedures following relaxation of strict regulation on elective surgeries, resulting the market growth in the post-pandemic era.
However, the market in Japan’s is expected to rise in demand due to the increase in purchasing demand during the pandemic owing to the continuation of cardiovascular surgical procedures given the exemption from stringent curbs for elective surgeries, as such the market is anticipated to grow moderately in the post-pandemic period.
Market Segment
In 2022, the Electrocardiogram (ECG) segment hold the largest market share growth during the forecast period
Cardiovascular Devices Market is bifurcated into Electrocardiogram (ECG), Remote Cardiac Monitoring, and Other Diagnostic and Monitoring Devices. Among these, the Electrocardiogram is influencing a higher growth rate over the forecast period. The electrical signals from the heart are recorded by electrocardiogram devices. This test is simple and painless that is used to detect heart problems and monitor heart problems. Such diagnostic features are helpful in the cardiovascular process and are expected to grow the market. ECGs are becoming more approved particularly in the home healthcare market. Moreover, an increasing incidence of cardiovascular diseases wherein long-term ECG monitoring is important, with the introduction of wireless ECG, doctors can remotely monitor and diagnose on the time, additionally fueling the market growth.
Remote Cardiac Monitoring has anticipated the growth of the market during the forecast period. Remote patient monitoring enables the healthcare providers to manage acute and chronic conditions while decreasing the patient's travel cost and risk of infection. Remote cardiac monitoring is a method of communicating information from a patient's implantable rhythm management device to a physician's office.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan Cardiovascular Devices Market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- Boston Scientific Corporation
- Edwards Lifesciences
- Medtronic PLC
- Cardinal Health Inc.
- Biotronik
- Siemens Healthineers AG
- W. L. Gore & Associates, Inc.
- Canon Medical Systems Corporation
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In February 2021, Remo Care introduced Remo Cardia, this remote cardiac monitoring solution employs AI to track and assess patients' vital signs in real time owing to the market growth is as a result of such product launches.
- In October 2022, Japan Lifeline Co., Ltd launched the AT-Patch, a patch-type electrocardiogram (ECG) recorder. Additionally, the company announced the implementation of a five-year exclusive Japan distribution acceptance with IL GANG Medicare Co., Ltd. for the AT-Patch.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2019 to 2030. Spherical Insights has segmented the Japan Cardiovascular Devices Market based on the below-mentioned segments:
Japan Cardiovascular Devices Market, By Device Type
- By Diagnostic and Monitoring Devices
- Diagnostic and Monitoring Devices (Electrocardiogram (ECG)
- Remote Cardiac Monitoring
- Other Diagnostic and Monitoring Devices
- By Therapeutic and Surgical Devices
- Cardiac Assist Devices
- Cardiac Rhythm Management Devices
- Catheter, Grafts
- Heart Valves, Stents
- Other Therapeutic and Surgical Devices
Need help to buy this report?