Japan In-Vitro Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (Instruments, Reagents, Others), By Test Type (Radiology, Pathology, Others), and Japan In-Vitro Diagnostics Market Insights Forecasts to 2033
Industry: HealthcareJapan In-Vitro Diagnostics Market Insights Forecasts to 2033
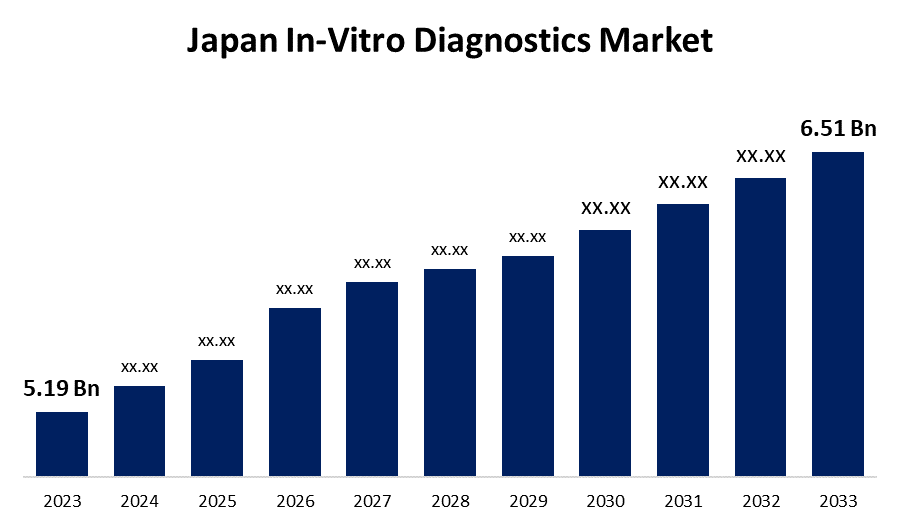
- The Japan In-Vitro Diagnostics Market Size was valued at USD 5.19 Billion in 2023.
- The Market Size is Growing at a CAGR of 2.29% from 2023 to 2033.
- The Japan In-Vitro Diagnostics Market Size is Expected to Reach USD 6.51 Billion By 2033.
Get more details on this report -
The Japan In-Vitro Diagnostics Market Size is expected to reach USD 6.51 Billion by 2033, at a CAGR of 2.29% during the forecast period 2023 to 2033.
Market Overview
Japan's medical device market is one of the largest, and its In-Vitro Diagnostics (IVD) market encompasses a wide range of tests, including clinical chemistry, immunoassays, microbiology, molecular diagnostics, and point-of-care testing. The Pharmaceuticals and Medical Devices Agency regulates IVD products in Japan. Furthermore, Japan is a market leader in diagnostic imaging, with a strong position due to advanced technology and expertise. The aging population and rising prevalence of neurological diseases, including Alzheimer's disease, are propelling the IVD market in Japan. To meet the increased demand for better neurological healthcare, new IVD and high modalities are being developed. These advancements will not only help the medical device industry grow, but will also allow for better treatment of patients suffering from neurological illnesses. Moreover, the industry is seeing remunerative growth potential due to an increased focus on research and development (R&D) and partnerships between diagnostics businesses and research institutions. In addition, the integration of big data and artificial intelligence (AI) in diagnostics, as well as the improvement of analytical capacities and personalization of care, are creating lucrative potential for market expansion.
Report Coverage
This research report categorizes the market for Japan in-vitro diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan in-vitro diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segments of the Japan in-vitro diagnostics market.
Japan In-Vitro Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 5.19 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 2.29% |
| 2033 Value Projection: | USD 6.51 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 115 |
| Segments covered: | By Product, By Test Type |
| Companies covered:: | Galderma S.A., Miraca Holdings Inc., Eurofins Scientific, BML, Inc., Forest Japan Medical Centre, H.U. Group, and other key companies. |
| Pitfalls & Challenges: | Covid-19 Empact,Challenges,Growth, Analysis. |
Get more details on this report -
Driving Factors
The diagnosis of chronic and infectious diseases fuels the market growth during the forecast period. Infectious disease diagnosis refers to the laboratory test which is performed by the skilled lab technicians under the guidance of physicians. The diagnostic lab market is growing due to rise in prevalence of numerous chronic and infectious disease such as influenza, HIV, AIDS, COVID-19, and hepatitis. Furthermore, diagnostic lab centers are getting funds from private & public organization to enhance the infrastructure of testing center on account of the increasing infectious and chronic diseases. Along with this, the technological advancements in the field of infectious diseases diagnostics are also contributing to the market growth. Inflating awareness among individuals for early diagnosis and management of infectious disease as well as growing number of information technology system to launch advanced diagnostic devices are expected to drive the Japan diagnostic labs market in the forecast period.
Restraining Factors
The market's growth would be hampered by the shortage of skilled laboratory workers, the high cost of high-end molecular diagnostics and in vitro diagnostics devices, and the onerous premarket approval (PMA) and IVD labeling requirements.
Market Segment
- In 2023, the instruments segment accounted for the largest revenue share over the forecast period.
Based on the product, the Japan in-vitro diagnostics market is segmented into instruments, reagents and others. Among these, the instruments segment has the largest revenue share over the forecast period. Rising demand is being driven by factors such as increased demand for laboratory automation and efficiency, technological advancements in diagnostic instruments, which result in faster, more accurate, and higher throughput testing, and a growing emphasis on specialized testing that necessitates dedicated equipment, which is expected to be the primary driver of segment growth.
- In 2023, the pathology segment accounted for the largest revenue share over the forecast period.
Based on the test type, the Japan in-vitro diagnostics market is segmented into radiology, pathology and others. Among these, the pathology segment has the largest revenue share over the forecast period. Demand for diagnostic labs is expected to rise as people become more aware of medical testing and early disease detection. Pathology offers alternatives by allowing for home and laboratory testing. The growing awareness of the benefits of using diagnostic labs in the country, as well as the ability to select the testing type based on the patient's preferences, will contribute to segmental growth over the forecast period.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan in-vitro diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Galderma S.A.
- Miraca Holdings Inc.
- Eurofins Scientific
- BML, Inc.
- Forest Japan Medical Centre
- H.U. Group
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts country revenue from 2022 to 2033. Spherical Insights has segmented the Japan in-vitro diagnostics market based on the below-mentioned segments:
Japan In-Vitro Diagnostics Market, By Product
- Instruments
- Reagents
- Others
Japan In-Vitro Diagnostics Market, By Test Type
- Radiology
- Pathology
- Others
Need help to buy this report?