Japan Real World Evidence Solutions Market Size, Share, and COVID-19 Impact Analysis, By Component (Datasets and Consulting & Analytics), and By End-User (Pharmaceutical, Biotechnology, and Medical Device Companies), and Japan Real World Evidence Solutions Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareJapan Real World Evidence Solutions Market Insights Forecasts to 2033
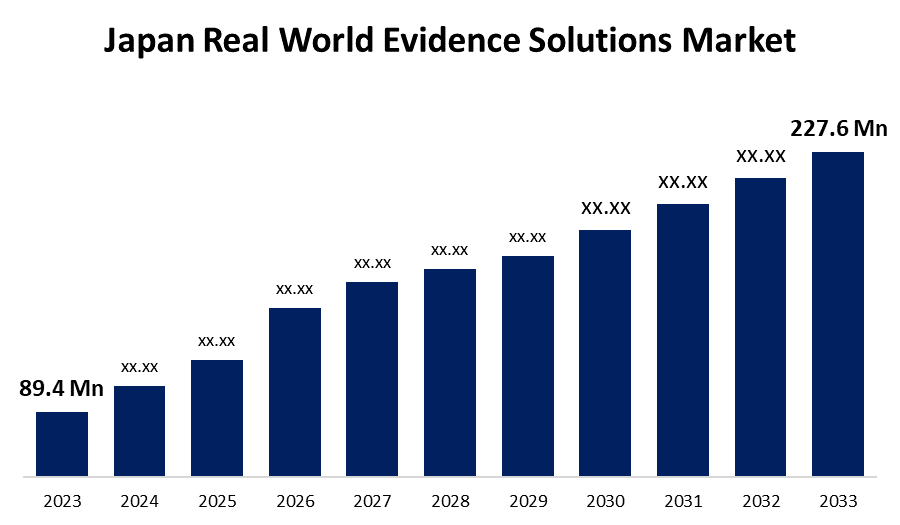
- The Japan Real World Evidence Solutions Market Size was valued at USD 89.4 Million in 2023
- The Market is Growing at a CAGR of 9.80% from 2023 to 2033
- The Japan Real World Evidence Solutions Market Size is Expected to Reach USD 227.6 Million by 2033
Get more details on this report -
The Japan Real World Evidence Solutions Market is Anticipated to Reach USD 227.6 Million by 2033, growing at a CAGR of 9.80% from 2023 to 2033.
Market Overview
The Japanese healthcare sector that uses real-world data to produce insights and evidence for medical treatments, medication research, and healthcare decision-making is known as the Japan real-world evidence (RWE) solutions market. Additionally, real-world data is used to assess treatment outcomes and assist patients in receiving optimal care. Patient-provided data, mobile devices, registries, healthcare claims and billing operating systems, electronic health records (EHR), and other resources are analyzed to create RWE. Both experimental and observational research, as well as databases, may be its source. Furthermore, the need for more medical equipment in Japan is being driven by the country's aging population, which requires ongoing care over an extended period. The market need for cutting-edge medical healthcare products and therapies is rising in Japan as a result of the country's aging population and changing lifestyle, which mostly causes chronic diseases like diabetes. This has helped the Japanese market for real-world proof solutions to expand.
Report Coverage
This research report categorizes the market for the Japan real world evidence solutions market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan real world evidence solutions market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan real world evidence solutions market.
Japan Real World Evidence Solutions Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 89.4 Million |
| Forecast Period: | 2023 – 2033 |
| Forecast Period CAGR 2023 – 2033 : | 9.80% |
| 023 – 2033 Value Projection: | USD 227.6 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 255 |
| Tables, Charts & Figures: | 132 |
| Segments covered: | By Component, By End-User |
| Companies covered:: | IQVIA Holdings Inc. International Business Machines Corp (IBM) Thermo Fisher Scientific Inc. PAREXEL International Corporation PerkinElmer, Inc. Icon PLC Oracle Corporation Others |
| Pitfalls & Challenges: | COVID-19 Empact,Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The growth of the real-world evidence solutions market in Japan is driven by the rising incidence of chronic diseases, delays in drug development and the consequent increase in development costs, the rising focus on personalized healthcare, a shift toward value-based care, and the growing adoption of real-world evidence solutions in drug development and commercialization. Furthermore, in Japan, emerging opportunities and an increasing focus on end-to-end RWE services are expected to offer significant growth potential for players operating in the real-world evidence solutions market. Additionally, in Japan, the healthcare industry has witnessed tremendous change in the last few years, with a shift toward value-based care spurred by healthcare organizations' efforts to reduce costs, improve outcomes, and enhance returns on investment. Various organizations in Japan are focusing on population health and driving improved outcomes through value-based care delivery models. Companies in Japan must possess strong evidence lifecycle management capabilities to prove and articulate their value propositions.
Restraining Factors
The Japanese real world evidence solutions market is subject to a number of restraints, notwithstanding its potential for expansion. One major obstacle is the high expense of growing real world evidence solutions, which may have an impact on the end product's price.
Market Segmentation
The Japan real world evidence solutions market share is classified into component and end user.
- The datasets segment is expected to hold a significant market share through the forecast period.
The Japan real world evidence solutions market is segmented by component into datasets and consulting & analytics. Among these, the datasets segment is expected to hold a significant market share through the forecast period. Real-world data (RWD) about patient outcomes in real-world settings are gathered from a variety of sources. Retrospective studies frequently use this data to provide real-world evidence (RWE). In addition to the clinical and financial effects on patients and healthcare systems, RWE provides insightful information about unmet requirements.
- The pharmaceutical segment is expected to hold a significant market share through the forecast period.
The Japan real world evidence solutions market is segmented by end-user into pharmaceutical, biotechnology, and medical device companies. Among these, the pharmaceutical segment is expected to hold a significant market share through the forecast period. As a result of the increased necessity to prevent drug recalls and evaluate drug performance in real-world situations, RWE studies are becoming more and more important in drug development and approvals.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan real world evidence solutions market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA Holdings Inc.
- International Business Machines Corp (IBM)
- Thermo Fisher Scientific Inc.
- PAREXEL International Corporation
- PerkinElmer, Inc.
- Icon PLC
- Oracle Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Japan Real world evidence solutions Market based on the below-mentioned segments:
Japan Real World Evidence Solutions Market, By Component
- Datasets
- Consulting & Analytics
Japan Real World Evidence Solutions Market, By End-User
- Pharmaceutical
- Biotechnology
- Medical Device Companies
Need help to buy this report?