Japan Regulatory Affairs Market Size, Share, and COVID-19 Impact Analysis, By Services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Applications, and Other Services), By Category (Drugs, Biologics, and Medical Devices), and Japan Regulatory Affairs Market Insights, Industry Trend, Forecasts to 2033.
Industry: HealthcareJapan Regulatory Affairs Market Insights Forecasts to 2033
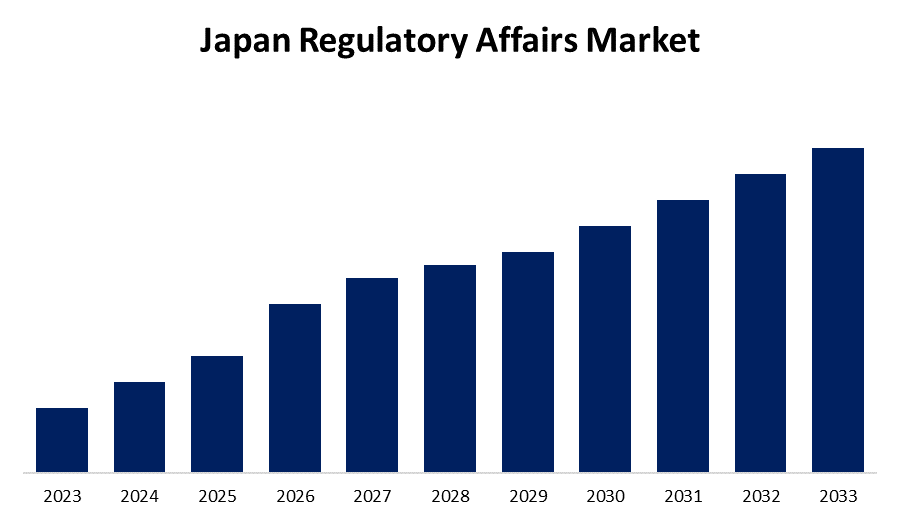
- The Market Size is Growing at a CAGR of 7.2% from 2023 to 2033
- The Japan Regulatory Affairs Market Size is expected to hold significant shares by 2033
Get more details on this report -
Japan Regulatory Affairs Market is anticipated to hold a significant share by 2033, Growing at a CAGR of 7.2% from 2023 to 2033.
Market Overview
The field of regulatory affairs developed out of the government's desire to safeguard public health by monitoring the efficacy and safety of products in a variety of industries, such as pharmaceuticals, veterinary care, medical devices, pesticides, agrochemicals, cosmetics, and complementary and alternative medicine, as well as the companies in charge of product development, testing, manufacturing, and marketing, who want to make sure their products are safe and contribute meaningfully to public health and welfare. A new category of experts has emerged to manage these regulatory affairs on behalf of companies. The rapid need for medications to address the illness increased the need for regulatory procedures to establish a secure environment where numerous clinical trials can be carried out. The proliferation of novel diseases that require efficient treatments and vaccines, as well as the growth of emerging fields such as immunotherapies, orphan drugs, personalized medicines, combination therapies, and specialty therapies, as well as shifts in regulatory requirements and the need for governing strategies to maintain quality, efficiency, and safety.
Report Coverage
This research report categorizes the market for the Japan regulatory affairs market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the regulatory affairs market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the regulatory affairs market.
Japan Regulatory Affairs Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 7.2% |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 95 |
| Segments covered: | By Services, By Category |
| Companies covered:: | Genpact, ICON plc, WuXi AppTec, Charles River Laboratories, Pharmalex GmbH, CRITERIUM, INC., Labcorp Drug Development, Medpace, Freyr, and other key companies. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis |
Get more details on this report -
Driving Factors
It is anticipated that an increase in companies pursuing inorganic expansion strategies including alliances, mergers, and acquisitions will boost the regulatory affairs market's expansion. Throughout the projected period, growing financial burdens on individuals and a growing number of government initiatives in different nations are expected to drive market expansion. In addition, substantial R&D expenditures and developments in the healthcare industry are important drivers of market growth. Furthermore, the necessity for third-party services in regulatory issues is being driven by the growing demand in the life sciences sector to cut costs. Moreover, during the projected period, the development of effective software to handle records related to regulatory issues is anticipated to further accelerate market expansion.
Restraining Factors
Regulatory compliance necessitates large resources, such as financial investments and qualified staff, which can put a strain on budgets and impede the expansion of smaller enterprises.
Market Segmentation
The Japan regulatory affairs market share is classified into services and category.
- The regulatory writing & publishing segment is expected to hold the largest market share through the forecast period.
The Japan regulatory affairs market is segmented by services into regulatory consulting, legal representation, regulatory writing & publishing, product registration & clinical trial applications, and other services. Among these, the regulatory writing & publishing segment is expected to hold the largest market share through the forecast period. Mid- and large-sized biopharmaceutical and medical device companies are increasingly outsourcing these activities to other countries. Big pharmaceutical and medical device firms outsource regulatory affairs tasks, such as publishing and developing regulations, which helps them highlight their core expertise and efficiently manage their internal resources.
- The medical devices segment dominates the market with the largest market share over the predicted period.
The Japan regulatory affairs market is segmented by category into drugs, biologics, and medical devices. Among these, the medical devices segment dominates the market with the largest market share over the predicted period. Over the next several years, it is anticipated that this market sector will continue to grow rapidly and maintain its leading position. The enormous rise observed in the market is mostly attributable to the growing tendency to outsource various medical device-related tasks. These large market companies are using outsourcing facilities to boost the concentration on their core capabilities. The market is expected to develop as a result of several factors, including the complexity of drug-device combinations, growing demand for wearable medical devices, and quick advances in material sciences.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan regulatory affairs market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Genpact
- ICON plc
- WuXi AppTec
- Charles River Laboratories
- Pharmalex GmbH
- CRITERIUM, INC.
- Labcorp Drug Development
- Medpace
- Freyr
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In August 2022, the acquisition of OneSource Regulatory & OneSource Regulatory Technology was announced by ProPharma Group. The acquisition intends to bring together top-notch services and solutions for the life sciences industry.
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Japan Regulatory Affairs Market based on the below-mentioned segments
Japan Regulatory Affairs Market, By Services
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Other Services
Japan Regulatory Affairs Market, By Category
- Drugs
- Biologics
- Medical Devices
Need help to buy this report?