Global Medical Device CRO Market Size, Share, and COVID-19 Impact Analysis, By Phase (Preclinical and Clinical), By Services (Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/Assurance, Bio-statistics, Investigator Payments, Laboratory, Patient & Site Recruitment, Technology, and Others), By Device (MedTech Devices, Diagnostic Devices, Handheld Devices, and Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Medical Device CRO Market Insights Forecasts to 2033
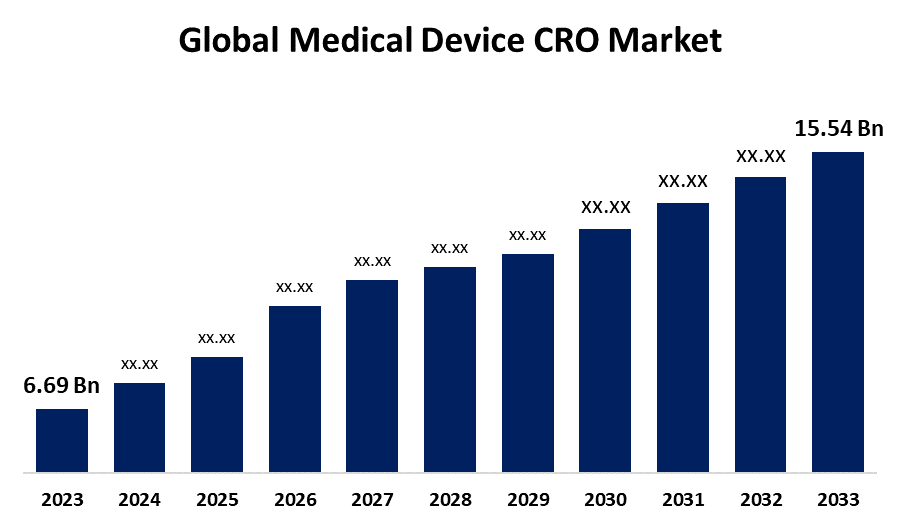
- The Global Medical Device CRO Market Size was Valued at USD 6.69 Billion in 2023
- The Market Size is Growing at a CAGR of 8.79% from 2023 to 2033
- The Worldwide Medical Device CRO Market Size is Expected to Reach USD 15.54 Billion by 2033
- Europe is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Medical Device CRO Market Size is Anticipated to Exceed USD 15.54 Billion by 2033, Growing at a CAGR of 8.79% from 2023 to 2033.
Market Overview
A Contract Research Organization (CRO) is a corporation that offers clinical trial services to pharmaceutical, biotechnology, and medical device companies. There are various types of CROs, but widespread CRO offerings to the medical device industry include regulatory affairs, clinical trial planning, site selection and initiation, recruitment support, clinical monitoring, data management, trial logistics, biostatistics, medical writing, and project management. Medical device CROs are intended to lower expenses for corporations researching innovative medications and pharmaceuticals for special markets. They aim to streamline entry into drug markets and also development, as huge pharmaceutical corporations no longer need to handle everything in-house. CROs also help foundations, research institutes, and universities, as well as government bodies such as the NIH, EMA, etc.
According to the World Health Organization, around two million medical gadgets are currently available worldwide. It is worth noting that, since 2020, the US Food and Drug Administration has approved almost 105 medical devices, with over 35 receiving FDA approval per year. Furthermore, medical devices are expected to account for more than 40% of the worldwide medtech industry, with the share increasing steadily in the coming years.
The significant medical device CRO market share of the companies included in this report is Avania, Charles River Laboratories, CROMSOURCE, CSSi Life Sciences, Eurofins Medical Testing, IQVIA, Medpace, Qserve, NAMSA, ICON, plc, Syneos Health, WuXi, AppTec, Promedica International, and others.
Report Coverage
This research report categorizes the market for the medical device CRO market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the medical device CRO market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the medical device CRO market.
Global Medical Device CRO Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 6.69 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.79% |
| 2033 Value Projection: | USD 15.54 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 244 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Phase, By Services, By Device, By Region |
| Companies covered:: | Avania, Charles River Laboratories, CROMSOURCE, CSSi Life Sciences, Eurofins Medical Testing, IQVIA, Medpace, Qserve, NAMSA, ICON, plc, Syneos Health, WuXi, AppTec, Promedica International, and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The market expansion can be ascribed to an increase in the number of medical device-specific clinical trials, rising demand for innovative medical devices, and medical device firms' increased focus on lowering research costs. The increased needs for novel technologies, along with the desire to make devices more patient-friendly, are projected to strengthen the medical device market during the projection period. The medical device contract research organization (CRO) industry is expanding significantly as a result of increased access to superior technological resources. It saves both time and money. CROs are gaining importance in the healthcare and medical fields.
Restraining Factors
Major restricting issues include the high cost of medical equipment, regulatory changes, and the complexity of payment policies for illness treatment. These issues are expected to restrict market growth over the target period.
Market Segmentation
The medical device CRO market share is classified intophase, services, and device.
- The clinical segment dominates the market with the highest market share through the forecast period.
Based on the phase, the medical device CRO market is categorized into preclinical and clinical. Among these, the clinical segment dominates the market with the highest market share through the forecast period. The medical device CROs increasing number of clinical trials focusing on medical devices is driving the growth. This is primarily due to medical device makers' increased emphasis on lowering research costs. Furthermore, the segment's expansion is supported by a vast supply of clinical-stage medical devices.
- The clinical monitoring segment is predicted to account for the largest revenue share through the forecast period.
Based on the services, the medical device CRO market is categorized into project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/assurance, bio-statistics, investigator payments, laboratory, patient & site recruitment, technology, and others. Among these, the clinical monitoring segment is predicted to account for the largest revenue share through the forecast period. The medical device CRO market is predicted to maintain its position during the projected period due to the significant number of clinical trials conducted and the increasing need for clinical trial monitoring. The development of devices such as smart analytics and real-time is expected to improve clinical monitoring data, hence supporting sector market growth.
- The diagnostic devices segment is predicted to dominate the medical device CRO market during the forecast period.
Based on the device, the medical device CRO market is categorized into medtech devices, diagnostic devices, handheld devices, and others. Among these, the diagnostic devices segment is predicted to dominate the medical device CRO market during the forecast period. The increasing prevalence of diseases throughout the world is driving demand for CRO operations for diagnostic devices. The large number of people suffering from these diseases is predicted to raise demand for devices for diagnosis and encourage diagnostic device research activities by CROs.
Regional Segment Analysis of the Medical Device CRO Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
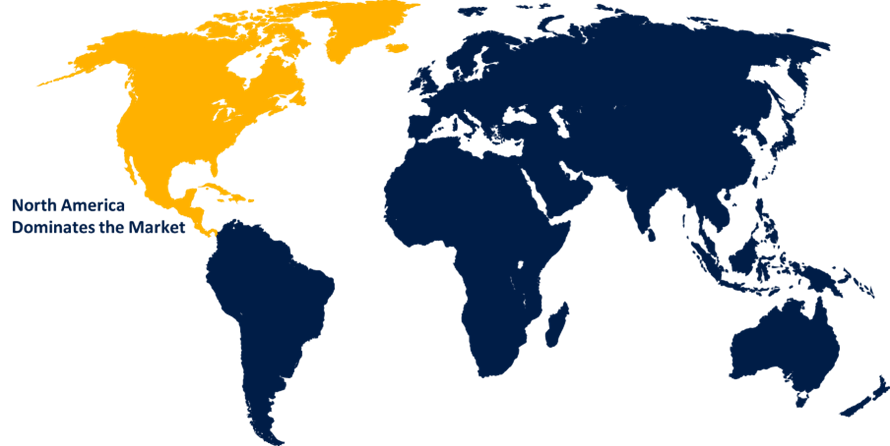
North America is anticipated to hold the largest share of the medical device CRO market over the predicted timeframe.
Get more details on this report -
North America is projected to hold the largest share of the medical device CRO market over the forecast period.The region's dominance is due in part to the presence of major pharmaceutical companies, overall drug research activity, and a strong healthcare infrastructure. Pharmaceutical companies have expanded their emphasis on outsourcing clinical studies to address various illness problems. In addition, these organizations are increasing their investment in R&D. One of the key factors driving market expansion in North America is the rapid development of medical devices to fulfill the increasing need for effective healthcare. Furthermore, rising demand for lower-cost medical equipment is expected to drive market expansion throughout the forecast period.
Europe is expected to grow at the fastest CAGR growth in the medical device CRO market during the forecast period. Modern technologies and well-established infrastructure have resulted in improved healthcare facilities and patient care. Players intending to enter the European market must be proficient in the regulatory procedures and services available in the various EU member states. For instance, In November 2023, Medpace received Meditech, a medical device CRO established in the Netherlands, to increase its presence in the European market.
The Asia Pacific medical device CRO market is expected to grow significantly over the forecast period. The market is anticipated to be driven by a variety of factors, including regulatory changes, higher cost savings, increasing device complexity, and a growing number of medical device research firms in the region. Furthermore, the growing trend of outsourcing research services from developed economies to emerging economies such as India and China, combined with the availability of a trained workforce at a lower cost than in the United States and Europe, is projected to drive market expansion.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the medical device CRO market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Avania
- Charles River Laboratories
- CROMSOURCE
- CSSi Life Sciences
- Eurofins Medical Testing
- IQVIA
- Medpace
- Qserve
- NAMSA
- ICON, plc
- Syneos Health
- WuXi
- AppTec
- Promedica International
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In June 2024, Lindus Health developed a comprehensive contract research organization (CRO) solution tailored exclusively to medical device clinical trials. The 'All-in-One Medical Device CRO' blends typical CRO services with Lindus Health's expertise in medical device clinical trials.
- In January 2024, MCRA, a prominent privately held independent clinical research organization (CRO) and consultancy firm for medical devices, diagnostics, and biologics, revealed its expansion into Europe, with three new locations in London, the United Kingdom, Winterthur, Switzerland, and Eschborn, Germany.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the medical device CRO market based on the below-mentioned segments:
Global Medical Device CRO Market, By Phase
- Preclinical
- Clinical
Global Medical Device CRO Market, By Services
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Patient & Site Recruitment
- Technology
- Others
Global Medical Device CRO Market, By Device
- MedTech Devices
- Diagnostic Devices
- Handheld Devices
- Others
Global Medical Device CRO Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1 Global Medical Device CRO Market Size, Share, and COVID-19 Impact Analysis, By Phase (Preclinical and Clinical), By Services (Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/Assurance, Bio-statistics, Investigator Payments, Laboratory, Patient & Site Recruitment, Technology, and Others), By Device (MedTech Devices, Diagnostic Devices, Handheld Devices, and Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033 1. What is the CAGR of the global medical device CRO market over the forecast period?The global medical device CRO market is to expand at 8.79% during the forecast period.
-
2. Which region is expected to hold the highest share in the global medical device CRO market?The North America region is expected to hold the largest share of the global medical device CRO market.
-
3. Who are the top key players in the medical device CRO market?The key players in the global medical device CRO market are Avania, Charles River Laboratories, CROMSOURCE, CSSi Life Sciences, Eurofins Medical Testing, IQVIA, Medpace, Qserve, NAMSA, ICON, plc, Syneos Health, WuXi, AppTec, Promedica International, and others.
Need help to buy this report?