Global Medical Device Package Validation Market Size, Share, and COVID-19 Impact Analysis, By Testing Type (Physical Testing, Microbial Testing, Chemical Testing, and Visual Testing), By Device Class (Class I Devices, Class II Devices, and Class III Devices), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Medical Device Package Validation Market Insights Forecasts to 2033
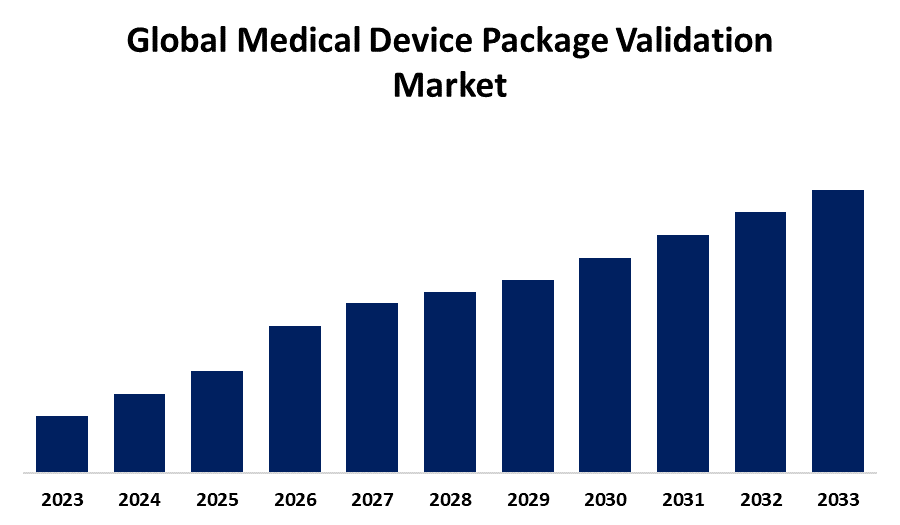
- The Medical Device Package Validation Market Size is Expected to hold a significant share by 2033
- The Market Size is Growing at a CAGR of 8.59% from 2023 to 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Medical Device Package Validation Market Size is Expected to Hold a Significant Share by 2033, at a CAGR of 8.59% during the forecast period 2023-2033.
Market Overview
The process of determining and managing the materials and processing variables that influence a packaged device's capacity to satisfy acceptance standards is known as medical device package validation. Identifying the optimal windows for each significant variable allows process control and assurance to meet the device package requirements. A medical device package validation helps sure that products and packaging systems are efficient and safe from production to delivery and storage to final user, that can be a pharmacist at the local drugstore or a doctor in a hospital. The market for medical device package validation is driven by stringent standards that ensure the safety, efficacy, and integrity of medical equipment throughout its lifecycle. The regulatory scrutiny drives the growth of the medical device package validation market by compelling manufacturers to invest in strong systems that adhere to international standards.
Challenges
The main challenges are a lack of understanding of the properties of packing materials and difficulties with documentation and record-keeping, which are projected to hinder the expansion of the medical device package validation market.
Report Coverage
This research report categorizes the medical device package validation market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the medical device package validation market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the medical device package validation market.
Global Medical Device Package Validation Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.59% |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 248 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Testing Type, By Device Class, By Region |
| Companies covered:: | Glatfelter Corporation, Berry Global Inc, Amcor Plc, Wetspak, Life Science Outsourcing, Inc., Pro-Tech Design & Manufacturing, WuXi AppTec Medical Device Testing, Nelson Labs, Keystone Package Testing, Eurofins Scientific, UL Solutions, SteriPack Contract Manufacturing, DDL, Inc., and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
A key element to improving patient safety and building consumer trust is the medical device packaging validation. Consumers growing concerns about the spread of infectious illnesses drive the demand for medical device package validation market. A greater focus on patient safety and infection prevention, stringent regulatory requirements for study packaging, and technological advancements in medical devices that demand specialized packaging to maintain the sterility and integrity of complex healthcare are the main factors driving the demand for medical device package validation market.
Restraining Factors
Strict rules, high costs, low knowledge, and resource limitations restrict innovation and compliance in the medical device package validation market, particularly for smaller manufacturers.
Market Segmentation
The medical device package validation market share is classified into testing type and device class.
- The physical testing segment is estimated to hold the largest market revenue share through the projected period.
Based on the testing type, the medical device package validation market is classified into physical testing, microbial testing, chemical testing, and visual testing. Among these, the physical testing segment is estimated to hold the largest market revenue share through the projected period. The term "physical testing" is related to the evaluation of the mechanical properties, robustness, and integrity of packing materials and systems in a variety of environments.
- The class II devices segment is anticipated to hold the largest market share through the forecast period.
Based on the device class, the medical device package validation market is divided into class I devices, class II devices, and class III devices. Among these, the class II devices segment is anticipated to hold the largest market share through the forecast period. Class II devices, such as infusion pumps and diagnostic imaging equipment, are medical devices with intermediate risk levels that require thorough validation to ensure that the packaging maintains product integrity and sterility.
Regional Segment Analysis of the Medical Device Package Validation Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
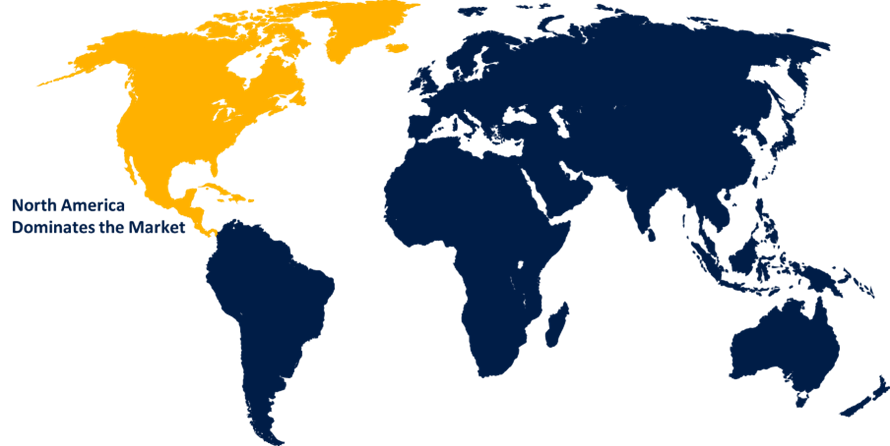
North America is anticipated to hold the largest share of the medical device package validation market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the medical device package validation market over the predicted timeframe. Strong healthcare infrastructure, stringent regulatory frameworks including FDA standards, and the existence of significant medical device manufacturers are all factors contributing to the market for North America. Due to these qualities ensuring adherence to stringent quality and safety criteria, the region is in great need of comprehensive package validation services. Moreover, the growth of package validation solutions in North America is facilitated by the ongoing advancements in medical technology as well as the presence of a significant market.
Asia Pacific is expected to grow at the fastest CAGR growth of the medical device package validation market during the forecast period. The rapid development of the regional market is driven by rising healthcare costs, increasing acceptance of medical devices, and rising patient safety and regulatory compliance awareness. The need for medical device package validation services is growing as a result of significant investments being made in industrial capabilities and healthcare infrastructure in China, India, and Japan.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the medical device package validation market along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Glatfelter Corporation
- Berry Global Inc
- Amcor Plc
- Wetspak
- Life Science Outsourcing, Inc.
- Pro-Tech Design & Manufacturing
- WuXi AppTec Medical Device Testing
- Nelson Labs
- Keystone Package Testing
- Eurofins Scientific
- UL Solutions
- SteriPack Contract Manufacturing
- DDL, Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In February 2024, Glatfelter Corporation and Berry Global Inc. announced their plans to split and merge their Health, Hygiene, and Specialties Global Nonwovens and Films (HHNF) business.
- In January 2024, Amcor Plc announced that it is increasing its thermoforming production capacity to satisfy the increasing demand from customers in the consumer health, pharmaceutical, and medical sectors in North America.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the medical device package validation market based on the below-mentioned segments:
Global Medical Device Package Validation Market, By Testing Type
- Physical Testing
- Microbial Testing
- Chemical Testing
- Visual Testing
Global Medical Device Package Validation Market By Device Class
- Class I Devices
- Class II Devices
- Class III Devices
Global Medical Device Package Validation Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the medical device package validation market over the forecast period?The medical device package validation market is projected to expand at a CAGR of 8.59% during the forecast period.
-
2. What is the market size of the medical device package validation market?The Global Medical Device Package Validation Market is Expected to Hold a Significant Share by 2033, at a CAGR of 8.59% during the forecast period 2023-2033.
-
3. Which region holds the largest share of the medical device package validation market?North America is anticipated to hold the largest share of the medical device package validation market over the predicted timeframe.
Need help to buy this report?