Global Medical Device Regulatory Affairs Market Size, Share, and COVID-19 Impact Analysis, By Regulatory Phase (Pre-Market, Post-Market), By Service (Product Registration & Clinical Trial Applications, Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing) By Type (Therapeutics and Diagnostic), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033.
Industry: HealthcareGlobal Medical Device Regulatory Affairs Market Insights Forecasts to 2033
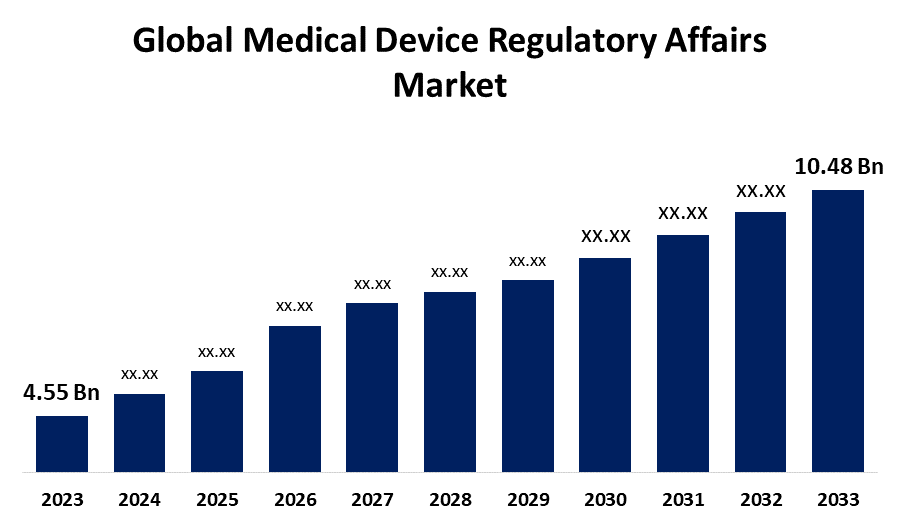
- The Global Medical Device Regulatory Affairs Market Size was Valued at USD 4.55 Billion in 2023
- The Market Size is Growing at a CAGR of 8.70% from 2023 to 2033
- The Worldwide Medical Device Regulatory Affairs Market Size is Expected to Reach USD 10.48 Billion by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Medical Device Regulatory Affairs Market Size is Anticipated to Exceed USD 10.48 Billion by 2033, Growing at a CAGR of 8.70% from 2023 to 2033.
Market Overview
Medical device regulatory affairs are an important and varied subject in the healthcare business that ensures that medical devices meet the regulatory criteria established by governments and international organizations. This discipline is critical in protecting public health because it ensures that medical devices, ranging from basic bandages to complicated diagnostic equipment, are safe and effective for their intended purpose before they are made available to patients and healthcare professionals. Regulatory affairs professionals must navigate the complex and often rigorous requirements set by authorities such as the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other national regulatory bodies. Their job begins early in the product development cycle when they give strategic direction on regulatory requirements that must be satisfied, confirming that design, testing, and manufacturing processes meet the appropriate standards.
Report Coverage
This research report categorizes the market for the global medical device regulatory affairs market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global medical device regulatory affairs market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global medical device regulatory affairs market.
Global Medical Device Regulatory Affairs Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 4.55 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.70% |
| 2033 Value Projection: | USD 10.48 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 261 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Regulatory Phase, By Service, By Type, and By Region |
| Companies covered:: | TUV SUD, SGS, UL (Underwriters Laboratories), Bureau Veritas, Medpace, PAREXEL, NAMSA, Charles River, Regulatory Compliance Associates, RAPS (Regulatory Affairs Professionals Society), CRB, HCL Technologies, Eurofins Scientific, Catalent, Celerion, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Numerous factors will boost the growth of the medical device regulatory affairs market globally. Medical device regulatory affairs verify that medical equipment complies with all regulations so that firms may receive market approval and access international markets. These bodies aid in maintaining strict safety regulations which lowers the possibility of patient injury and guarantees that medical devices work as planned. They provide a framework for managing compliance and minimizing risks protecting the organization from legal concerns and financial monetary penalties. Also, professionals in regulatory affairs assist businesses in navigating complex rules that accelerate the entrance of cutting-edge medical equipment into the market.
Restraining Factors
Several restraining factors will inhibit the growth of the medical device regulatory affairs market globally. Compliance with strict regulations can prove time-consuming and expensive causing delays in product development and increased expenses. Global market access becomes even more challenging because of differences in national regulatory standards that demand multiple paperwork and different approaches to compliance. Noncompliance with guidelines can have serious implications like product recalls, penalties, or legal action that undermines a company's brand and financial stability.
Market Segmentation
The global medical device regulatory affairs market share is classified into regulatory phase, service, and type.
- The post-market segment is expected to hold the largest share of the global medical device regulatory affairs market during the forecast period.
Based on the regulatory phase, the global medical device regulatory affairs market is divided into pre-market and post-market. Among these, the post-market segment is expected to hold the largest share of the global medical device regulatory affairs market during the forecast period. To make sure that medical devices are safe and effective throughout their lives regulatory agencies are putting a greater focus on post-market surveillance that includes stricter surveillance and reporting standards. Companies that are expanding into worldwide markets must comply with various post-market standards across different areas raising the demand for specialized post-market regulatory services. Also, the growing emphasis on ensuring the patient's safety and well-being throughout the life-cycle of the medical device is driving the growth post-market segment.
- The regulatory consultant segment is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period.
Based on the service, the global medical device regulatory affairs market is divided into product registration & clinical trial applications, regulatory consulting, legal representation, and regulatory writing & publishing. Among these, regulatory consulting is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period. Companies are seeking professional guidance to overcome the complex requirements of regulatory obligations that vary by region. Regulatory consultants offer comprehensive evaluations and strategic direction to assist businesses in adhering to a variety of constantly evolving requirements. This aids businesses in managing the risks connected to adhering to regulations, such as potential delays, rejections, and legal difficulties.
- The therapeutic segment is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period.
Based on the type, the global medical device regulatory affairs market is divided into therapeutics and diagnostic. Among these, the therapeutic segment is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period. Because of the rising frequency of chronic illnesses, aging populations, and technological breakthroughs in medicine, there is an increasing demand for novel therapeutic devices. This raises the necessity for regulatory assistance to bring innovative therapeutic devices to market. Also, significant investment is spent on the research and development of innovative therapeutic devices, especially in areas like oncology, cardiology, and neurology.
Regional Segment Analysis of the Global Medical Device Regulatory Affairs Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the global medical device regulatory affairs market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of the global medical device regulatory affairs market over the predicted timeframe. The United States is one of the world's major markets for medical devices with a well-developed regulatory framework and a significant amount of medical device activity. North America, led by the U.S. Food and Drug Administration, has a highly developed and serious regulatory framework that guarantees device safety and effectiveness. This stringent regulatory environment increases the demand for expert regulatory affairs services. Also, the United States is a vital hub for medical device innovation with multiple top corporations and research organizations that drive continuous regulatory activities for various medical products.
Asia Pacific is expected to grow at the fastest pace in the global medical device regulatory affairs market during the forecast period. The region's healthcare and medical device industries are expanding quickly due to factors like rising healthcare costs, population expansion, and increased public awareness of cutting-edge medical technology. Fast-growing economies like China and India, are making significant investments in healthcare infrastructure and technology so the need for medical equipment and regulatory support services is increased by this economic growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global medical device regulatory affairs market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- TUV SUD
- SGS
- UL (Underwriters Laboratories)
- Bureau Veritas
- Medpace
- PAREXEL
- NAMSA
- Charles River
- Regulatory Compliance Associates
- RAPS (Regulatory Affairs Professionals Society)
- CRB
- HCL Technologies
- Eurofins Scientific
- Catalent
- Celerion
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In September 2022, AmerisourceBergen Corporation completes the acquisition of PharmaLex Holding GmbH, a significant participant in the life sciences services market, from AUCTUS Capital Partners AG, enhancing the company's standing and competencies in the sector.
- In August 2022, PharmaLex Group expanded its worldwide portfolio with the merger of DRA Consulting, a Finland-based company that specializes in pharmacovigilance, market access, quality, regulatory services, and complete solutions for more than 300 customers.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global medical device regulatory affairs market based on the below-mentioned segments:
Global Medical Device Regulatory Affairs Market, By Regulatory Phase
- Pre-Market
- Post-Market
Global Medical Device Regulatory Affairs Market, By Service
- Product Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
Global Medical Device Regulatory Affairs Market, By Type
- Therapeutics
- Diagnostic
Global Medical Device Regulatory Affairs Market, Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. Which are the key companies that are currently operating within the market?TUV SUD, SGS, UL (Underwriters Laboratories), Bureau Veritas, Medpace, PAREXEL, NAMSA, Charles River, Regulatory Compliance Associates, RAPS (Regulatory Affairs Professionals Society), CRB, HCL Technologies, Eurofins Scientific, Catalent, Celerion, and Others.
-
2. What is the size of the global medical device regulatory affairs market?The Global Medical Device Regulatory Affairs Market is expected to grow from USD 4.55 Billion in 2023 to USD 10.48 Billion by 2033, at a CAGR of 8.70% during the forecast period 2023-2033.
-
3. Which region is holding the largest share of the market?North America is anticipated to hold the largest share of the global medical device regulatory affairs market over the predicted timeframe.
Need help to buy this report?