Global Medical Device Testing Service Market Size, Share, and COVID-19 Impact Analysis, By Service (Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation), By Phase (Preclinical, Clinical), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2021 - 2030
Industry: HealthcareGlobal Medical Device Testing Service Market Insights Forecasts to 2030
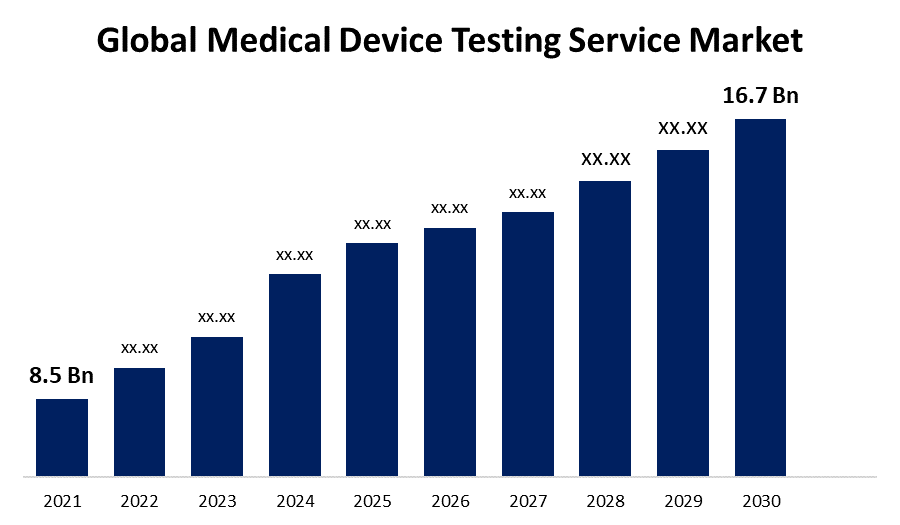
- The Medical Device Testing Service Market was valued at USD 8.5 Billion in 2021.
- The Market is growing at a CAGR of 9.3% from 2022 to 2030
- The Worldwide Medical Device Testing Service Market is expected to reach USD 16.7 Billion by 2030
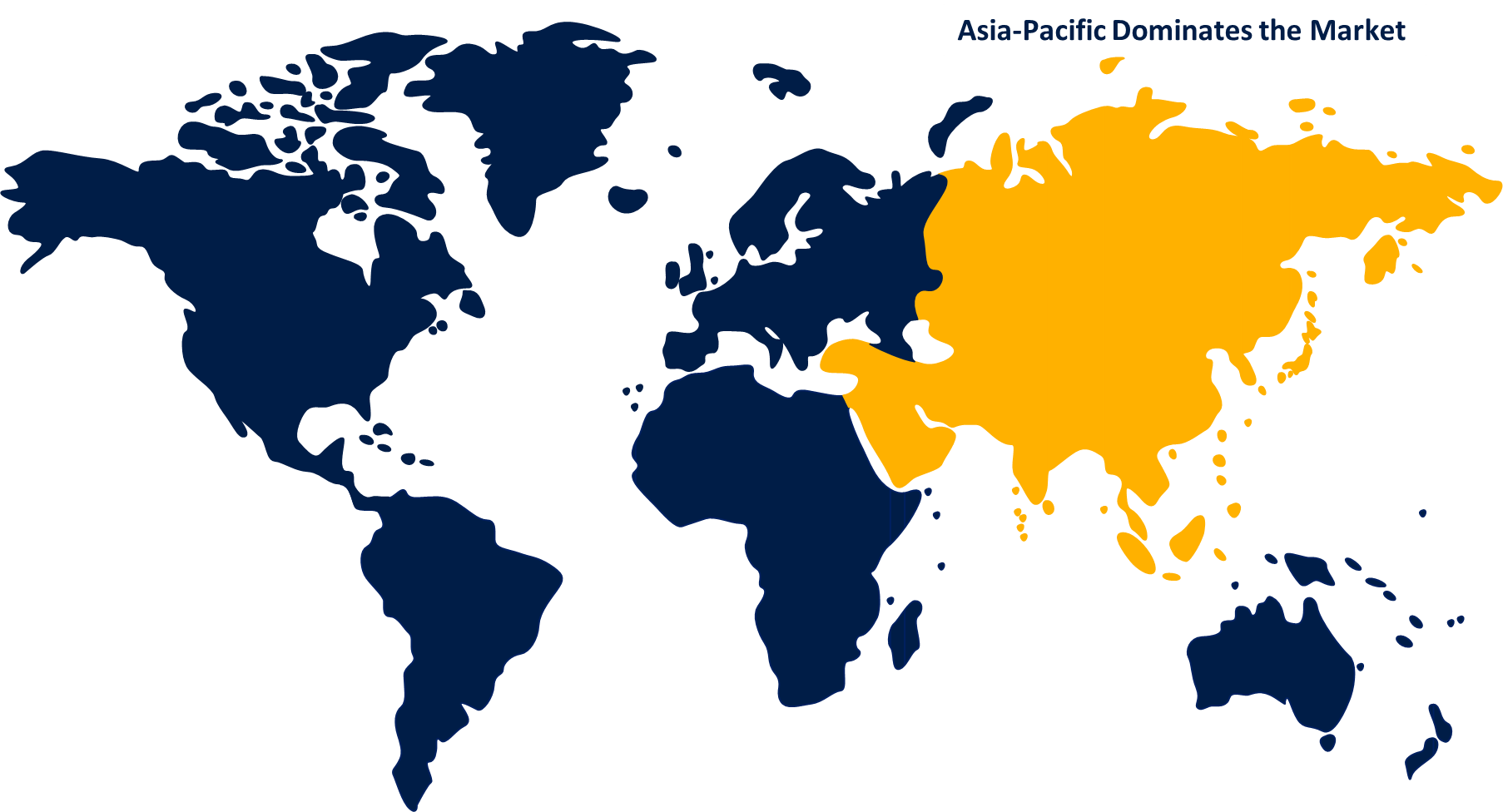
- Asia Pacific is expected to grow the fastest during the forecast period
Get more details on this report -
The Global Medical Device Testing Service Market is expected to reach USD 16.7 Billion by 2030, at a CAGR of 16.7% during the forecast period 2022 to 2030.
The process of proving a medical device's dependability and safety while in use is known as medical device testing. Extensive design validation testing is used in new product development. This covers performance testing, chemical analysis and toxicity testing, and occasionally human aspects or even clinical testing. Continuous quality assurance testing typically has fewer resources. Dimensional checks, some functional tests, and package verification are typically included. There are many different kinds of medical testing services on the market, including inspection services and certification services.
Impact of COVID 19 on Global Medical Device Testing Service Market
With the onset of COVID 19 pandemic, governments of various countries have implemented different measures to prevent the spread of the deadly virus such as lockdown measures and restricting import and export activities between various nations which has disrupted the supply chain thus impacted the global medical device testing service market. Not only this but also with the disruption of supplychain has resulted into the shortage of critical medical devices across the globe. Thus, various countries have implemented certain measures to work on these shortages by importing equipment like domestic manufacturing of medical devices. Furthermore, domestic manufacturing of important medical devices is anticipated to overcome the barriers in trade in order to ensure market stability as well as product stability.
Global Medical Device Testing Service Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2021 |
| Market Size in 2021: | USD 8.5 Billion |
| Forecast Period: | 2021-2030 |
| Forecast Period CAGR 2021-2030 : | 9.3% |
| 2030 Value Projection: | USD 16.7 Billion |
| Historical Data for: | 2017-2020 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Service, By Phase, By Region, and COVID-19 Impact Analysis |
| Companies covered:: | SGS SA, Euro fins Scientific, Pace Analytical Services LLC, ntertek Group Plc, WUXI APPTEC, TÜV SÜD AG, Sterigenics International LLC, American Preclinical Services, North American Science Associates, Inc., Charles River Laboratories International, Inc, Medical Device Testing Services, Toxikon, Inc |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Key Market Drivers
In the healthcare sector, verification and validation techniques are widely and extensively used. In general, validation determines whether a product has been used for its intended purpose and, as a result, whether usability standards have been met. Verification, on the other hand, determines whether a product has been developed to the given requirements. Design, process, and software verification and validation are the most typical methods of verification and validation for medical devices. Additionally, the size and complexity of medical gadgets are increasing, and they occasionally make use of sophisticated, manufactured plastics. This increases the significance of validation and verification (V&V). Better repeatability, fewer errors, less need for rework and redesign, quicker time to market, increased competitiveness, and lower production costs are the end results.
Key Market Challenges
The high cost associated with the medical devices and the barriers to the local development of medical devices in some of the medical devices in some of the regions will hamper the development of the growth of the global medical device testing market. Apart from this, the increasing competition in medical technology market as well as long lead time for overseas qualification is proven to be challenging to the market developments.
Market Segmentation
Services Insights
Microbiology & Sterility testing segment holds the highest market share over the forecast period
On the basis of services, the global medical device testing market is segmented into Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation. Among these, the microbiology and sterility testing segment holds the highest market share over the forecast period. Microbiology and sterility testing are done to eliminate or reduce the risk of contamination in the production procedure which led to infections in users or patients. The regulatory process for the devices could be delayed if such examination are not performed.
Phase Insights
Clinical segment is dominating the market with the highest market share over the forecast period
Based on the phase, the global medical device testing market is segmented into preclinical and clinical. Among these, the clinical segment is dominating the market with the highest market share over the forecast period. The price of the medical device testing services is comparatively higher than pre clinical devices which is one of the primary factors to determine the largest market in the clinical phase. Moreover, the testing of medical devices needs more time in each phase than it does in the preclinical stage and the volume of testing is also higher which is the another major factor that is contributing towards the segmental growth.
Regional Insights
Asia Pacific is dominating the market with the largest market share over the forecast period
Get more details on this report -
Asia Pacific is dominating the market with the largest market share over the forecast period. The growth is attributed to expansion of the international interest in Chinese as well as Indian markets which is driven by the strict product approval standards in China and development in healthcare infrastructure. Due to the strong presence of China in medical devices testing market, various product launches are happening to maintain compliance with international standards.
North America, on the other hand is witnessing the fastest market growth over the forecast period due to the rising complexity in product design and growing efforts made to reduce costs. Apart from this, the presence of strict regulatory bodies like FDA is also propelling the regional market growth. The rise in the manufacturing of medical devices in order to meet the high demand for efficient healthcare in this region is anticipated to boost the regional demand.
Recent Market Developments
- In 2021, TUV SUD has made an announcement that it would attend MedTech LIVE to demonstrate its ability as a one stop shop for medical device testing.
List of Key Companies
- SGS SA
- Euro fins Scientific
- Pace Analytical Services LLC
- Intertek Group Plc
- WUXI APPTEC
- TÜV SÜD AG
- Sterigenics International LLC
- American Preclinical Services
- North American Science Associates, Inc.
- Charles River Laboratories International, Inc
- Medical Device Testing Services
- Toxikon, Inc.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2019 to 2030. Spherical Insights has segmented the global Medical Device Testing Service Market based on the below-mentioned segments:
Medical Device Testing Service Market, System Analysis
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Testing
- Package Validation
Medical Device Testing Service Market, Phase Analysis
- Clinical
- Pre-clinical
Medical Device Testing Service Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the market size of Medical Device Testing Service Market?The global Medical Device Testing Service Market is expected to grow from USD 8.5 Billion in 2021 to USD 16.7 Billion by 2030, at a CAGR of 9.3% during the forecast period 2022-2030.
-
2. Who are the key market players of Medical Device Testing Service Market?Some of the key market players of Medical Device Testing Service Market are SGS SA; Euro fins Scientific; Pace Analytical Services LLC; Intertek Group Plc; WUXI APPTEC; TÜV SÜD AG; Sterigenics International LLC; American Preclinical Services; North American Science Associates, Inc.; Charles River Laboratories International, Inc; Medical Device Testing Services; Toxikon, Inc.
-
3. Which segment hold the largest market share?Clinical hold the largest market share is going to continue its dominance.
-
4. Which region is dominating the Medical Device Testing Service Market?North America is dominating the Medical Device Testing Service Market with the highest market share.
Need help to buy this report?