Global Pharmacovigilance and Drug Safety Software Market Size, Share, and COVID-19 Impact Analysis, By Functionality (Adverse Event Reporting Software, Signal Detection, and Others), By Delivery Mode (On-Demand and On-Premise), By End-User (Contract Research Organizations (CROs), Pharma and Biotech Companies, Business Process Outsourcing Firms, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Pharmacovigilance and Drug Safety Software Market Insights Forecasts to 2033
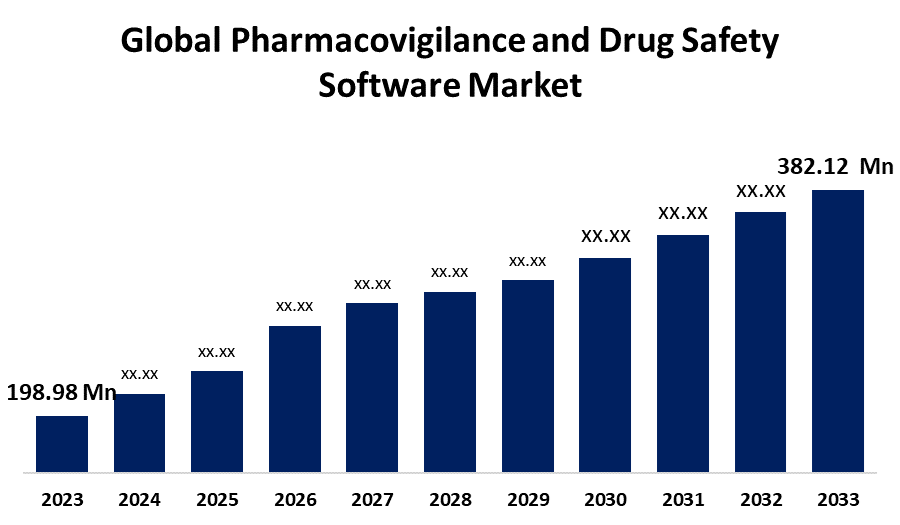
- The Global Pharmacovigilance and Drug Safety Software Market Size was Estimated at USD 198.98 Million in 2023
- The Market Size is Expected to Grow at a CAGR of around 6.74% from 2023 to 2033
- The Worldwide Pharmacovigilance and Drug Safety Software Market Size is Expected to Reach USD 382.12 Million by 2033
- Asia Pacific is predicted to grow at the fastest CAGR throughout the projection period
Get more details on this report -
The Global Pharmacovigilance and Drug Safety Software Market Size is Anticipated to Exceed USD 382.12 Million by 2033, Growing at a CAGR of 6.74% from 2023 to 2033. The global pharmacovigilance and drug safety software market is growing owing to rising adverse drug reactions, stringent regulations for drug development and approvals, and outsourcing companies adopting pharmacovigilance software. Top of Form
Market Overview
The pharmacovigilance and drug safety software market comprises digital tools for monitoring, analyzing, and reporting adverse drug reactions and safety-related information in pharmaceuticals and medical products, ensuring patient safety and regulatory compliance. Pharmacovigilance is the process of detecting, assessing, understanding, and preventing adverse drug reactions, severe adverse events, and severe events through data collection and drug safety software, benefiting patients, healthcare professionals, and manufacturers. The drug safety software stores reports of adverse events during clinical trials and post-marketing phases for drugs.
Adverse Drug reactions (ADR) are a major global health concern caused by undesired effects of drugs or treatment that prolong hospitalization and raise treatment expenditures, to overcome such conditions pharmacovigilance is an essential tool for assessing the safety of medicines. For instance, The Ministry of Health and Family Welfare, Government of India established the Pharmacovigilance Programme of India.
The rise in reporting adverse drug reactions to regulatory agencies worldwide is predicted to upsurge the demand for pharmacovigilance and data safety software. For example, the Therapeutic Goods Administration, an Australian regulatory authority, published a regulator performance report for 2021-2022. In 2020–21, there were 59,639 adverse event notifications; in 2021–22, there were 125,873 adverse event notifications.
Uppsala Monitoring Centre (UMC) is a WHO Collaborating Centre headquarters located in Sweden advancing pharmacovigilance globally, supporting the WHO Programme for International Drug Monitoring, and providing scientific, operational, educational, and communication support. The drug safety software that is extensively used by pharmaceutical companies, regulatory agencies, and healthcare professionals are Oracle Argus Safety, ArisG, Oracle Adverse Event Reporting System, ARGUS, PvNET, etc.
The pharmacovigilance and drug safety software market is expected to witness growth owing to the increasing demand for personalized medicine.
Report Coverage
This research report categorizes the market for the global pharmacovigilance and drug safety software market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global pharmacovigilance and drug safety software market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global pharmacovigilance and drug safety software market.
Global Pharmacovigilance and Drug Safety Software Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023 : | USD 198.98 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 6.74% |
| 2033 Value Projection: | USD 382.12 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 112 |
| Segments covered: | By Delivery Mode ,By Functionality, By Functionality and By Region |
| Companies covered:: | IQVIA, Genpact, Aris Global, Accenture, IBM, Capgemini, Paraxel International Corporation, Cognizant, United BioSource Corporation, Ennov Solutions Inc., Veeva Systems, Others, |
| Pitfalls & Challenges: | COVID-19 Empact,Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The pharmacovigilance and drug safety software market is growing due to the rising adoption of pharmacovigilance and data safety software by clinical research and outsourcing companies, the growing prevalence of chronic diseases, strict drug safety regulations, and the availability of cutting-edge technology. The market is additionally influenced by user-friendly interfaces and continuous advancements in software capabilities, making it easier for organizations to integrate these solutions. The rise in adverse drug reactions (ADRs) is propelling the global pharmacovigilance and drug safety software market. This demand for robust systems and software solutions is driven by stringent regulations and the need to monitor and report ADRs effectively. As healthcare expenditure increases globally, the focus is on improving patient safety and healthcare delivery quality. Pharmacovigilance and drug safety software are the primary needs for monitoring and managing adverse drug reactions, reducing the economic burden of medication-related harm, optimizing resources, and streamlining reporting and analysis processes. Therefore, all the factors contribute to the market expansion.
Restraining Factors
Inconsistent reporting of adverse drug reactions in low- and middle-income countries, owing to lack of necessary software, services, and training, is causing poor decisions and potential drug withdrawal, limiting the growth of the pharmacovigilance market.
Market Segmentation
The global pharmacovigilance and drug safety software market share is classified into functionality, delivery mode, and end-user.
- The adverse event reporting software segment accounted for the largest share in 2023 and is projected to grow at a remarkable CAGR during the projected timeframe.
Based on the functionality, the global pharmacovigilance and drug safety software market is categorized into adverse event reporting software, signal detection, and others. Among these, the adverse event reporting software segment accounted for the largest share in 2023 and is projected to grow at a CAGR during the projected timeframe. The segmental expansion is attributed to the provision of comprehensive data management features, allows the identification of data, enables the potential safety issues relevant to specific medications, accessibility to data, enhances the safety and quality of care, optimizes the ability to execute decisions, real-time monitoring, enhanced transparency and public trust, allows the early identification of ADR, regulatory compliance, patient data security.
- The on-demand segment held the largest market share of 50.10% in 2023 and is anticipated to grow at a significant CAGR throughout the projected timeframe.
Based on the delivery mode, the global pharmacovigilance and drug safety software market is categorized into on-demand and on-premise. Among these, the on-demand segment held the largest share in 2023 and is expected to grow at a significant CAGR throughout the projected timeframe. This segment growth is ascribed to streamlined accessibility, rapid acceptance by pharmaceutical companies, lower infrastructure cost, uniform flexibility, evaluates real-time data, enabling real-time stakeholder collaboration, faster decision-making, and comprehensive data analysis, while the SaaS model provides advanced pharmacovigilance tools previously exclusive to larger enterprises.
- The pharma and biotech companies segment dominated the market with 38.67% of the share in 2023 and is expected to grow at a substantial CAGR during the forecast period.
Based on the end-user, the global pharmacovigilance and drug safety software market is categorized as contract research organizations (CROs), pharma and biotech companies, business process outsourcing firms, and others. Among these, the pharma and biotech companies segment dominated the market with 38.67% of the share in 2023 and is expected to grow at a substantial CAGR during the forecast period. The growth of the segment is ascribed to rising consumption of pharmacovigilance software, advancements in technology including machine learning and artificial intelligence, enhanced accuracy of data and analysis, growing complexity of drug discovery and clinical trials, rising screening services, an increasing trend in the molecular study and focused on post-marketing surveillance studies, R&D activities, and customized to meet the standards of regulatory compliance.
Regional Segment Analysis of the Global Pharmacovigilance and Drug Safety Software Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
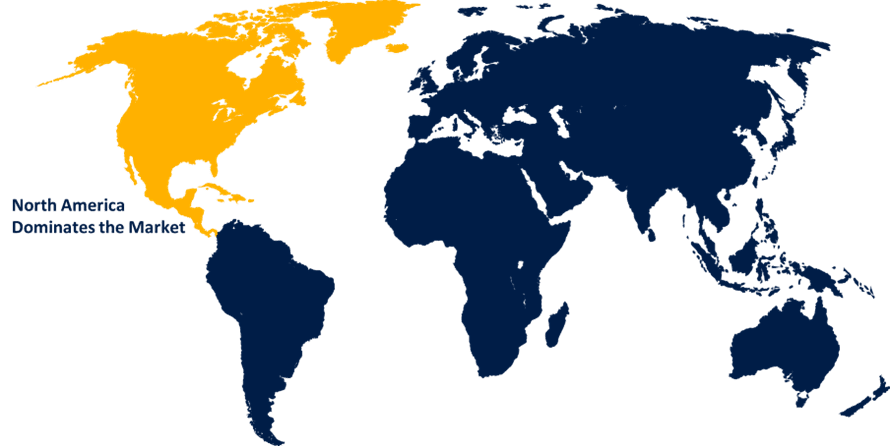
North America is anticipated to hold the largest share of the global pharmacovigilance and drug safety software market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global pharmacovigilance and drug safety software market over the forecast period.This is owing to government-aided initiatives. The open FDA initiative and Mini-Sentinel project, which provide active surveillance systems, are expected to boost usage rates. The increasing demand for effective drug safety solutions, strict regulations, the prevalence of chronic diseases, clinical trials, and medication approvals all of which are fueled by the many CROs that provide outsourcing services are the main drivers of the region's commercial gains.
Asia Pacific is anticipated to grow at the fastest CAGR throughout the projected timeframe. Asia Pacific businesses provide cost-cutting benefits, which encourage more clinical trials and an emphasis on pharmacovigilance and drug safety software. Public safety awareness, strict government controls, and an increase in adverse drug responses have made outsourcing centers including Singapore, South Korea, and Taiwan widely recognized.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global pharmacovigilance and drug safety software market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA
- Genpact
- Aris Global
- Accenture
- IBM
- Capgemini
- Paraxel International Corporation
- Cognizant
- United BioSource Corporation
- Ennov Solutions Inc.
- Veeva Systems
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In August 2024, Shri Narendra Modi launched 'Digital India', an indigenously developed ADRMS software for the Pharmacovigilance Programme of India (PvPI), with the Ministers of Health & Family Welfare and Chemicals and Fertilizers. PvPI's ADRMS software is India's first medical product safety database, enabling users to report adverse events related to medicines and medical devices.
- In February 2024, The FDA utilized an AI named Information Visualization Platform (InfoViP) to enhance its pharmacovigilance efforts by reviewing adverse event reports and standardizing Risk Evaluation and Mitigation Strategies data. InfoViP utilizes natural language processing to visualize temporal data, extract clinical concepts from case safety reports, classify reports based on information quality, and enable causality assessments using assessable ICSRs.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the global pharmacovigilance and drug safety software market based on the below-mentioned segments:
Global Pharmacovigilance and Drug Safety Software Market, By Functionality
- Adverse Event Reporting Software
- Signal Detection
- Other Safety Assessment
Global Pharmacovigilance and Drug Safety Software, By Delivery Mode
- On-Demand
- On-Premise
Global Pharmacovigilance and Drug Safety Software Market, By End-User
- Clinical Research Organizations (CROs)
- Pharma and Biotech Companies
- Business Process Outsourcing Firms
- Others
Global Pharmacovigilance and Drug Safety Software Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the global pharmacovigilance and drug safety software market?The global pharmacovigilance and drug safety software market is projected to expand at 6.74% during the forecast period.
-
2. Who are the top key players in the global pharmacovigilance and drug safety software market?The key players in the global pharmacovigilance and drug safety software market are IQVIA, Genpact, Aris Global, Accenture, IBM, Capgemini, Paraxel International Corporation, Cognizant, United BioSource Corporation, Ennov Solutions Inc., Veeva Systems, and others.
-
3. Which region holds the largest share of the market?North America is anticipated to hold the largest share of the global pharmacovigilance and drug safety software market over the predicted timeframe.
Need help to buy this report?