Global Point of Care Coagulation Testing Products Market Size, Share, and COVID-19 Impact Analysis, By Type (Prothrombin Time Testing Products, Activated Clotting Time (ACT/APTT) Testing Products, and Others), By Product Type (Instruments and Consumables), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Point of Care Coagulation Testing Products Market Insights Forecasts to 2033
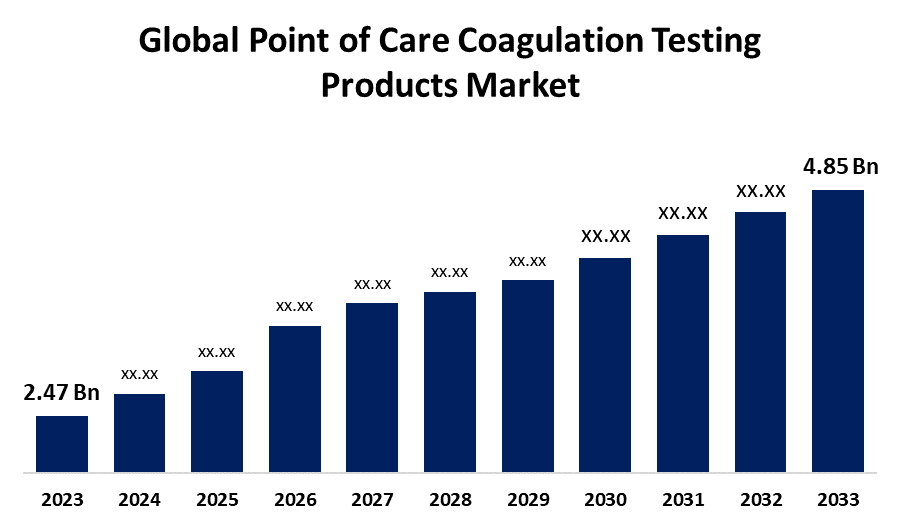
- The Global Point of Care Coagulation Testing Products Market Size was Valued at USD 2.47 Billion in 2023
- The Market Size is Growing at a CAGR of 6.98% from 2023 to 2033
- The Worldwide Point of Care Coagulation Testing Products Market Size is Expected to Reach USD 4.85 Billion by 2033
- Asia-Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Point of Care Coagulation Testing Products Market Size is Anticipated to Exceed USD 4.85 Billion by 2033, Growing at a CAGR of 6.98% from 2023 to 2033.
Market Overview
Point of care coagulation testing products are the devices that measure clotting parameters of small blood samples for diagnosis of the disorder, thereby facilitating treatment monitoring. These tests play an important role in detecting bleeding disorders and monitoring anticoagulant drugs. They are used for the determination of the coagulation status of patients taking anticoagulant medications. Prothrombin time testing, activated clotting time (act/aptt) testing, and activated partial thromboplastin clotting time are the various tests performed to measure the time taken by plasma/ whole blood cells to clot the blood. As per the report of the World Bleeding Disorders Registry (WBDR), among 11,374 individuals with hemophilia, only 30% of adults and 32% of children with severe hemophilia across 115 treatment centers in 44 countries, received prophylactic treatment in the year 2021. There is a rising emphasis on early detection of blood-related diseases and increased research funding, awareness campaigns, and subsidies for vital medical devices such as point-of-care coagulation testing products by the governments. The technological advancements in these testing products lead to the creation of increasingly precise, dependable, and user-friendly point of care coagulation testing products like refined sensors, automation, integration with electronic health records (EHRs), and seamless connectivity to smartphones or tablets. Hence, healthcare professionals are more confident in the use of these devices because of their increased accuracy and ease of use.
Report Coverage
This research report categorizes the market for the global point of care coagulation testing products market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the point of care coagulation testing products market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the point of care coagulation testing products market.
Global Point of Care Coagulation Testing Products Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 2.47 Billion |
| Forecast Period: | 2023 to 2033 |
| Forecast Period CAGR 2023 to 2033 : | 6.98% |
| 2033 Value Projection: | USD 4.85 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 125 |
| Segments covered: | By Type, By Product Type, By Region and COVID-19 Impact Analysis |
| Companies covered:: | Abbott Laboratories, F. Hoffman-La Roche AG, Medtronic Plc., Siemens Healthcare AG, Koninklijke Philips N.V., HemoSonics, LLC, Haemonetics Corp, Werfen, Micropoint Bioscience, Inc., Helena Laboratories, ThermoFisher Sceintific Inc., Sysmex Corporation, Nihon Kohden Corporation, Alere Inc., and others Key vedors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The increasing prevalence of blood clotting disorders and rising incidence of cardiovascular illnesses and cancer emphasizes the need for point of care coagulation monitoring in patients is driving the global point of care coagulation testing products market. The significant consumption of anticoagulant drugs driving the need for coagulation testing procedures for routine monitoring of blood clotting leads to drive the point-of-care (POC) coagulation testing market. The development of automated point of care coagulation testing products mediates rapid testing with minimal intervention which positively affects the market growth. The rising government initiatives for suppressing blood-related diseases, growing adoption of point of care testing products as well as constant product and technological advances led to drive the market growth. There is increasing support for the development of accurate, convenient, easy-to-use, affordable, equitably available, in-home POCTs that produce immediate results, empowering patients and home caregivers to diagnose, manage, enhance their adherence to medical treatments, and more efficiently engage their physicians. In addition, the improved healthcare infrastructure is contributing to market growth.
Restraining Factors
The stringent regulatory framework owing to maintain accuracy and precision of the testing results and high cost of product paont of care analyzer are anticipated to restrain the global point of care coagulation testing products market.
Market Segmentation
The point of care coagulation testing products market share is classified into type and product type.
- The prothrombin time testing products segment accounted for the largest revenue share of the global point of care coagulation testing products market in 2023.
Based on the type, the global point of care coagulation testing products market is categorized into prothrombin time testing products, activated clotting time (ACT/APTT) testing products, and others. Among these, the prothrombin time testing products segment accounted for the largest revenue share of the global point of care coagulation testing products market in 2023. Prothrombin time testing products are extensively used for screening for extrinsic factor deficiency, monitoring oral anticoagulant therapy and quantitative determination of the extrinsic coagulation factors. It is routinely used in clinical practice to evaluate the coagulation status of patients receiving vitamin K antagonists. The widespread application for detecting and diagnosing several bleeding and excessive clotting disorders and to monitor the efficiency of numerous anticoagulants upsurges the market demand in the prothrombin time testing products segment.
- The instruments segment dominated the market with the largest revenue share in 2023.
Based on the product type, the global point of care coagulation testing products market is categorized into instruments and consumables. Among these, the instruments segment dominated the market with the largest revenue share in 2023. The automation in the instruments possesses the benefits of high volume testing, better reproducibility, user flexibility, time and cost reduction, as well as better precision with quality controls. The integration with EHR (electronic health records) enhances efficiency and mediates informed decision-making. Thus, growing emphasis on technology advancements and constant improvement leads to driving market growth.
Regional Segment Analysis of the Point of Care Coagulation Testing Products Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
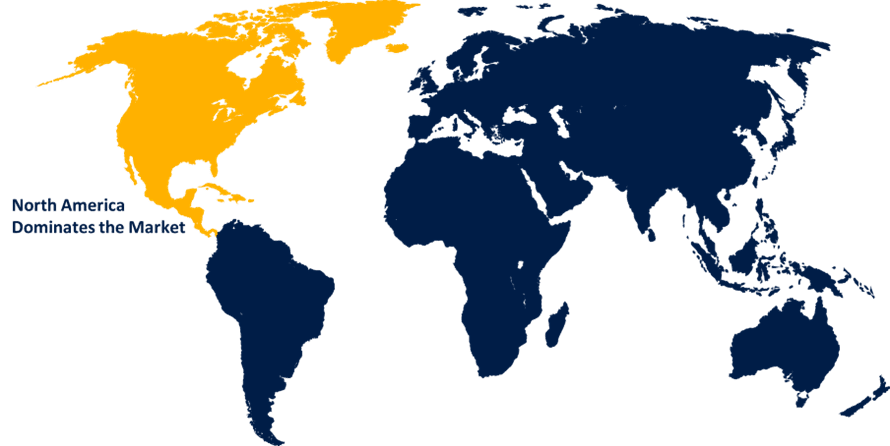
North America is anticipated to hold the largest share of the point of care coagulation testing products market over the predicted timeframe.
Get more details on this report -
North America is projected to hold the largest share of the point of care coagulation testing products market over the forecast period. The growing emphasis on preventive healthcare and the capabilities of point of care coagulation testing products for facilitating the management of blood disorders are driving the market in the region. Further, the growing investments in R&D fueling the innovation of products in testing technology with high accuracy and efficiency are driving the market growth in the region. Further, the increasing cases of hemophilia patients in the US and rising awareness about coagulation disorders are anticipated to drive the market demand.
Asia-Pacific is expected to grow at the fastest CAGR growth of the global point of care coagulation testing products market during the forecast period. The introduction of advanced coagulation testing devices, an increase in healthcare expenditure, and changes in dynamics in the in vitro diagnostic industry are all factors responsible for driving the market in the region. Further, the adoption of new technologies among hospitals and diagnostic laboratories is fueling the market growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global point of care coagulation testing products market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- F. Hoffman-La Roche AG
- Medtronic Plc.
- Siemens Healthcare AG
- Koninklijke Philips N.V.
- HemoSonics, LLC
- Haemonetics Corp
- Werfen
- Micropoint Bioscience, Inc.
- Helena Laboratories
- ThermoFisher Sceintific Inc.
- Sysmex Corporation
- Nihon Kohden Corporation
- Alere Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In April 2024, HemoSonics, a medical device company focused on acute bleeding management, announced it had received Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the expanded use of arterial blood samples with its Quantra QStat Cartridge. HemoSonics’ QStat Cartridge used with the Quantra Hemostasis Analyzer first received 510(k) clearance from the U.S. FDA in 2022 for use in venous whole blood samples.
- In October 2023, Universal Biosensors, Inc. announced that it had received the approval required for the sale of “Xprecia Prime 4U” directly to patients for self-testing in Europe. Xprecia Prime 4U is a portable, accurate, and easy-to-use device designed for fast and reliable prothrombin time (PT) results displayed in seconds and International Normalized Ratio (INR).
- In January 2022, Werfen announced the 510(k) clearance of the GEM Hemochron 100 whole blood hemostasis system by the US Food and Drug Administration (FDA). GEM Hemochron 100 system, delivers fast, actionable activated clotting time (ACT) results in minutes, informing patient-management decisions and helping improve workflow at the point of care (POC).
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global point of care coagulation testing products market based on the below-mentioned segments:
Global Point of Care Coagulation Testing Products Market, By Type
- Prothrombin Time Testing Products
- Activated Clotting Time (ACT/APTT) Testing Products
- Others
Global Point of Care Coagulation Testing Products Market, By Product Type
- Instruments
- Consumables
Global Point of Care Coagulation Testing Products Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the global point of care coagulation testing products market over the forecast period?The global point of care coagulation testing products market is projected to expand at a CAGR of 6.98% during the forecast period.
-
2. What is the projected market size & growth rate of the global point of care coagulation testing products market?The global point of care coagulation testing products market was valued at USD 2.47 Billion in 2023 and is projected to reach USD 4.85 Billion by 2033, growing at a CAGR of 6.98% from 2023 to 2033.
-
3. Which region is expected to hold the highest share in the global point of care coagulation testing products market?The North America region is expected to hold the highest share of the global point of care coagulation testing products market.
-
1. What is the CAGR of the global point of care coagulation testing products market over the forecast period?The global point of care coagulation testing products market is projected to expand at a CAGR of 6.98% during the forecast period.
-
2. What is the projected market size & growth rate of the global point of care coagulation testing products market?The global point of care coagulation testing products market was valued at USD 2.47 Billion in 2023 and is projected to reach USD 4.85 Billion by 2033, growing at a CAGR of 6.98% from 2023 to 2033.
-
3. Which region is expected to hold the highest share in the global point of care coagulation testing products market?The North America region is expected to hold the highest share of the global point of care coagulation testing products market.
Need help to buy this report?