Global Preeclampsia Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Blood Tests and Urine Analysis), By Product (Instruments and Consumables), By End-user (Hospitals, Specialty Clinics, Diagnostic Centers, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Preeclampsia Diagnostics Market Insights Forecasts to 2033
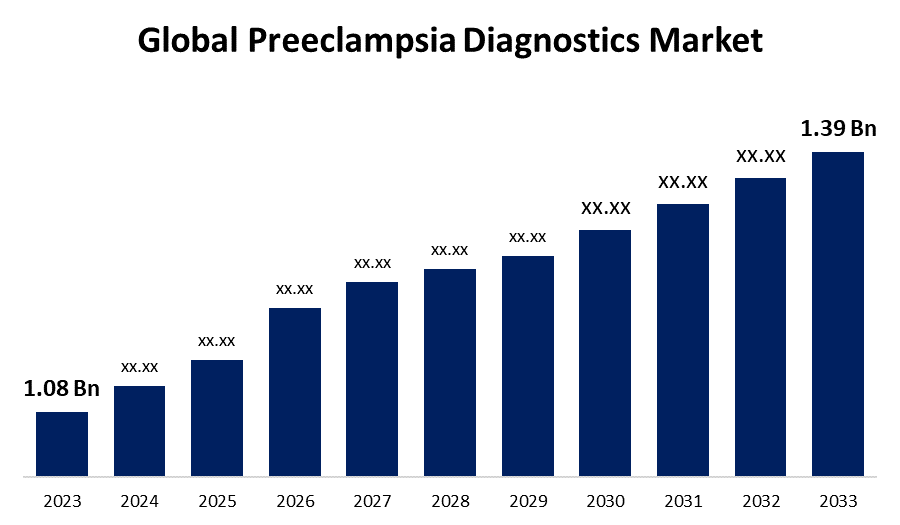
- The Global Preeclampsia Diagnostics Market Size was Valued at USD 1.08 Billion in 2023
- The Market Size is Growing at a CAGR of 2.56% from 2023 to 2033
- The Worldwide Preeclampsia Diagnostics Market Size is Expected to Reach USD 1.39 Billion by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Preeclampsia Diagnostics Market Size is Anticipated to Exceed USD 1.39 Billion by 2033, Growing at a CAGR of 2.56% from 2023 to 2033.
Market Overview
Preeclampsia, commonly referred to as toxemia, is a quickly developing condition defined by high blood pressure and a high amount of protein in pregnant women's urine. It is one of the pregnancy-related hypertension diseases that is speeding up the rates of maternal and perinatal morbidity and death among women in both developed and developing countries. A range of tests and tools are used in preeclampsia diagnostics to assist medical professionals in identifying and managing the condition in expecting patients. These diagnostic techniques support the early identification, monitoring, and evaluation of the problem's seriousness. The present delivery is the only option for treatment for preeclampsia, which greatly contributes to the problems related to preterm births, neonatal morbidity, and perinatal mortality. Early discovery and successful therapy of the disease depend on routine prenatal care and monitoring. The hypertension condition known as preeclampsia is still a major problem in obstetrics, which is driving up the need for precise and timely diagnostic techniques. The preeclampsia diagnostics market is growing as a result of factors such as rising healthcare costs, developing countries' improved healthcare infrastructure, and physicians' use of modern medical technology.
Report Coverage
This research report categorizes the market for the global preeclampsia diagnostics market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global preeclampsia diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global preeclampsia diagnostics market.
Global Preeclampsia Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023 : | USD 1.08 Billion |
| Forecast Period: | 2023 – 2033 |
| Forecast Period CAGR 2023 – 2033 : | 2.56% |
| 023 – 2033 Value Projection: | USD 1.39 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 235 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Test Type, By Product, By End-user, By Region |
| Companies covered:: | F. Hoffmann-La Roche Ltd, PerkinElmer Inc., Abbott Laboratories, DRG INSTRUMENTS GMBH, Thermo Fisher Scientific Inc., Diabetomics, Inc., Roche Diagnostic, Metabolomic Diagnostics Ltd., Sera Prognostics, The Progenix Group, Siemens Healthineers AG, Bayer AG, Quidel Corporation, Brahms GmbH, Others |
| Pitfalls & Challenges: | Covid-19 Impact, Challenge, Future,Growth and Analysis |
Get more details on this report -
Driving Factors
Preeclampsia identification tests will contribute to a decrease in mother and newborn deaths. The latest efforts by welfare associations and patient advocacy groups have raised awareness of preeclampsia. This continues to be a crucial component offering lucrative development prospects for novel test creation in light of the substantial unsatisfied need for preeclampsia diagnosis. Growing rates of the disease due to unhealthy lifestyle adoption and rising awareness among healthcare professionals and expectant mothers are expected to drive up demand for early screening, which can improve the market's growth in the forecast period. The goal of the expanding research and development of novel tests to identify biomarkers is to drive growth in the preeclampsia diagnostics market.
Restraining Factors
Preeclampsia in pregnant women is difficult to diagnose because a single increased blood pressure is insufficient. Many expectant women remain unsure of the term "preeclampsia" and its risks, despite its rising prevalence. In addition to putting women's health in danger, this lack of understanding about the condition's symptoms and effects increases the risk of newborn mortality.
Market Segmentation
The global preeclampsia diagnostics market share is classified into test type, product type, and end-user.
- The blood tests segment dominates the market with the largest market share through the forecast period.
Based on the test type, the global preeclampsia diagnostics market is categorized into blood tests and urine analysis. Among these, the blood tests segment dominates the market with the largest market share through the forecast period. Blood tests, using markers like soluble FMS-like tyrosine kinase-1 and placental growth factor, can accurately identify disorders associated with high blood pressure generated by pregnancy. Moreover, blood-based diagnostics enhance diagnostic consistency among various healthcare facilities by enabling remote laboratory testing and centralized analysis. Technological developments in blood testing continue to lower prices while improving detection capabilities. Blood tests are currently the recommended first-line diagnostic approach for preeclampsia screening and monitoring due to these advantages.
- The consumables segment is anticipated to grow at the fastest CAGR growth through the forecast period.
Based on the product type, the global preeclampsia diagnostics market is categorized into instruments and consumables. Among these, the consumables segment is anticipated to grow at the fastest CAGR growth through the forecast period. All preeclampsia diagnostic tests depend on consumables like reagents, kits, and other disposable products. In addition, single-use consumables are frequently needed for uniform and hygienic testing due to the practical limits of reusable instruments. Because these products are consumable, reagent suppliers and instrument makers can also profit from steady revenue streams. As diagnostic processes become more efficient and streamlined, consumables are designed for high-throughput applications that require no technical expertise and are easy to use.
- The hospitals segment accounted for the largest revenue share through the forecast period.
Based on the end-user, the global preeclampsia diagnostics market is categorized into hospitals, specialty clinics, diagnostic centers, and others. Among these, the hospitals segment accounted for the largest revenue share through the forecast period. The increasing number of in-hospital visits from toxemia patients is the reason for the segmental growth. Additionally, segmental expansion is anticipated to be driven by a growing number of hospitals in developing nations with better healthcare infrastructures and higher hospitalization rates.
Regional Segment Analysis of the Global Preeclampsia Diagnostics Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
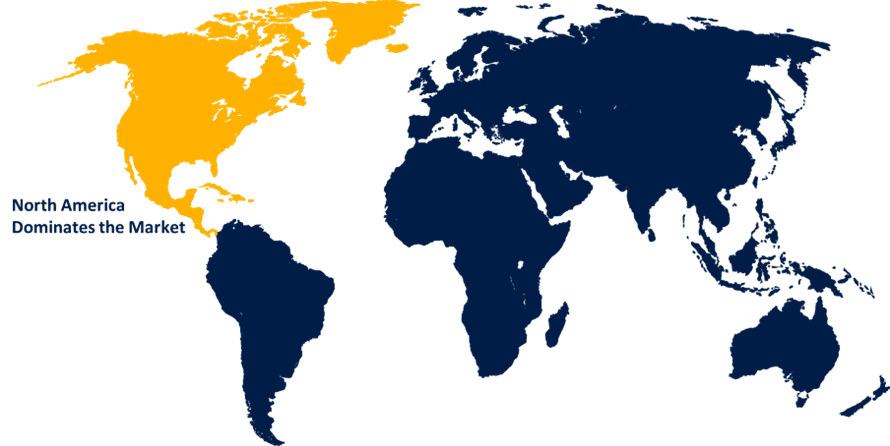
North America is anticipated to hold the largest share of the global preeclampsia diagnostics market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global preeclampsia diagnostics market over the predicted timeframe. The region's dominant position is largely attributed to its sophisticated healthcare system, strong R&D capacities, and high levels of maternal health awareness. Strong partnerships between academic institutions, healthcare providers, and business leaders also help North America accept cutting-edge diagnostic technologies more quickly. Due to the region's high healthcare costs, substantial presence of major diagnostics players, and rising preeclampsia prevalence. As the leader in diagnostics and medical devices, the United States controls the majority of the regional market.
Asia Pacific is expected to grow at the fastest CAGR growth of the global preeclampsia diagnostics market during the forecast period. Increasing investments in healthcare, a growing consciousness of maternal health, and the development of healthcare infrastructure in the region's nations are all contributing causes to this expansion. The region has seen a rise in the use of preeclampsia diagnostic tools due to its huge population, improved prenatal care, and lower rates of maternal death.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global preeclampsia diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- F. Hoffmann-La Roche Ltd
- PerkinElmer Inc.
- Abbott Laboratories
- DRG INSTRUMENTS GMBH
- Thermo Fisher Scientific Inc.
- Diabetomics, Inc.
- Roche Diagnostic
- Metabolomic Diagnostics Ltd.
- Sera Prognostics
- The Progenix Group
- Siemens Healthineers AG
- Bayer AG
- Quidel Corporation
- Brahms GmbH
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In January 2024, The laboratory services provider Labcorp launched a blood test for the assessment and management of severe preeclampsia risk that has been approved by the USFDA. One of TIME Magazine's Best Inventions of 2023, the test was created by Thermo Fisher Scientific and detects two biomarkers linked to preeclampsia.
- In July 2023, The USFDA approved a blood test developed by pharmaceutical and biotechnology company Thermo Fisher Scientific that accurately predicts preeclampsia in two weeks 96% of the time. Highly esteemed by experts such as Dr. Douglas Woelkers of the University of California, San Diego, it signifies a noteworthy advancement in the identification of preeclampsia.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global preeclampsia diagnostics market based on the below-mentioned segments:
Global Preeclampsia Diagnostics Market, By Test Type
- Blood Tests
- Urine Analysis
Global Preeclampsia Diagnostics Market, By Product Type
- Instruments
- Consumables
Global Preeclampsia Diagnostics Market, By End User
- Hospitals
- Specialty Clinics
- Diagnostic Centers
- Others
Global Preeclampsia Diagnostics Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Frequently Asked Questions (FAQ)
-
1. Which are the key companies that are currently operating within the market?F. Hoffmann-La Roche Ltd, PerkinElmer Inc., Abbott Laboratories, DRG INSTRUMENTS GMBH, Thermo Fisher Scientific Inc., Diabetomics, Inc., Roche Diagnostic, Metabolomic Diagnostics Ltd., Sera Prognostics, The Progenix Group, Siemens Healthineers AG, Bayer AG, Quidel Corporation, Brahms GmbH, and Others.
-
2. What is the size of the global preeclampsia diagnostics market?The Global Preeclampsia Diagnostics Market Size is Expected to Grow from USD 1.08 Billion in 2023 to USD 1.39 Billion by 2033, at a CAGR of 2.56% during the forecast period 2023-2033.
-
3. Which region is holding the largest share of the market?North America is anticipated to hold the largest share of the global preeclampsia diagnostics market over the predicted timeframe.
Need help to buy this report?