Global Radiopharmaceutical Preclinical CRO Market Size, Share, and COVID-19 Impact Analysis, By Radiopharmaceutical Types (PET Tracers, SPECT Tracers, and Therapeutic Radiopharmaceuticals) By Therapeutic Area (Oncology, Cardiology, Neurology, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Industry: HealthcareGlobal Radiopharmaceutical Preclinical CRO Market Insights Forecasts to 2033
- The Market Size is Growing at a CAGR of 8.47% from 2023 to 2033
- The Radiopharmaceutical Preclinical CRO Market Size is Expected to hold a significant share by 2033
- Asia Pacific is Expected to Grow the fastest during the forecast period.
Get more details on this report -
The Global Radiopharmaceutical Preclinical CRO Market Size is Expected to Hold a Significant Share by 2033, at a CAGR of 8.47% during the forecast period 2023-2033. The demonstrates significance of radiopharmaceutical CRO are in supporting neuroscience research and opening doors for potential improvements in the diagnosis and treatment of neurological conditions.
Market Overview
A crucial component of the pharmaceutical and healthcare sectors, the radiopharmaceutical preclinical CRO is dedicated to the creation and evaluation of radiopharmaceutical medications. This medication contains radioactive isotopes that are used for both therapeutic and diagnostic imaging. Radiopharmaceutical CRO perform research to assess the safety, effectiveness, and distribution of these specialty medications, offering crucial services in the early phases of drug development. The market for preclinical CRO for radiopharmaceuticals is the forefront of medical innovation. It is essential to the creation of next-generation treatments that have the potential to improve patient outcomes greatly. The increasing demand for oncology research is a primary factor driving the radiopharmaceutical preclinical CRO market. Effective radiopharmaceuticals are desperately needed for both diagnosis and treatment of cancer, which continues to rank among the leading causes of mortality.
Challenges
In addition to the encouraging trend, difficulties in the production of medicinal radiopharmaceuticals represent an important challenge. Research points to sporadic challenges in converting preclinical results into therapeutic radiopharmaceuticals that are clinically feasible. These issues are being addressed by CRO, such as Eurofins DiscoverX and Nuvisan, which highlight the necessity of smooth transitions from preclinical to clinical development.
Report Coverage
This research report categorizes the radiopharmaceutical preclinical CRO market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the radiopharmaceutical preclinical CRO market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the radiopharmaceutical preclinical CRO market.
Global Radiopharmaceutical Preclinical CRO Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.47% |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 241 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Radiopharmaceutical Types, By Therapeutic Area, By Region |
| Companies covered:: | Charles River Laboratories International, Inc., Pharmaron Beijing Co., Ltd., Novartis, Cardinal Health, Inc., Bioscan, Inc., Jubilant Radiopharma, Invicro (Konica Minolta), PerkinElmer, Inc., PAREXEL International Corporation, Siemens Healthineers, NorthStar Medical Radioisotopes, LLC, IBA Radiopharma Solutions, Eckert & Ziegler, Covance, Inc. (Labcorp Drug Development), and and Others |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The radiopharmaceuticals preclinical CRO market is a flourishing industry that is essential to the advancement of preclinical research in this field. The market for radiopharmaceutical preclinical CRO is propelled by developments in PET tracer research, the increasing need for studies in oncology and neurology, and CRO strategic partnerships. The primary factor driving the growth in the radiopharmaceutical preclinical CRO market is the growing interest in neurology research. The growing need for oncology research is one of the primary factors driving the radiopharmaceutical preclinical CRO market.
Restraining Factors
The market for radiopharmaceutical preclinical CROs is restricted by several difficulties, such as supply chain vulnerabilities, regulatory obstacles, high development costs, a lack of experience, fierce rivalry, technology constraints, patient safety issues, and extended delays.
Market Segmentation
The radiopharmaceutical preclinical CRO market share is classified into radiopharmaceutical type and therapeutic area.
- The PET tracers segment is estimated to hold the largest market revenue share through the projected period.
Based on the radiopharmaceutical type, the radiopharmaceutical preclinical CRO market is classified into PET tracers, SPECT tracers, and therapeutic radiopharmaceuticals. Among these, the PET tracers segment is estimated to hold the largest market revenue share through the projected period. PET tracers improved sensitivity and accuracy allow for more precise imaging and analysis during preclinical trials, which is especially helpful for studies in neurology and oncology.
- The oncology segment is anticipated to hold the largest market share through the forecast period.
Based on the therapeutic area, the radiopharmaceutical preclinical CRO market is divided into oncology, cardiology, neurology, and others. Among these, the oncology segment is anticipated to hold the largest market share through the forecast period. The growing regulatory requirements, oncology demand, and a greater focus on patient safety, and safety assessment are expanding quickly in the radiopharmaceutical preclinical CRO market.
Regional Segment Analysis of the Radiopharmaceutical Preclinical CRO Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
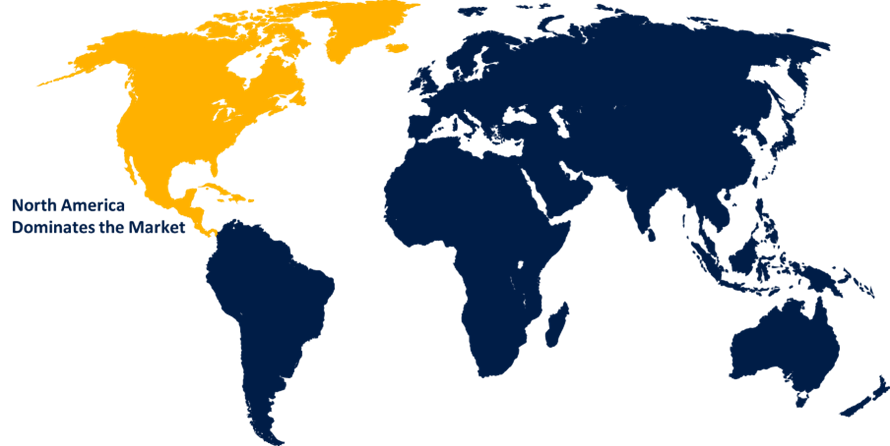
North America is anticipated to hold the largest share of the radiopharmaceutical preclinical CRO market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the radiopharmaceutical preclinical CRO market over the predicted timeframe. CRO invest in comprehensive safety assessments and compliance procedures due to the robust regulatory environment in North America, which focuses on safety and efficacy. North America is a key location for radiopharmaceutical preclinical CRO due to its leadership, infrastructure, and strict regulations. High-quality preclinical investigations are supported by the region sophisticated research infrastructure, which includes cutting-edge labs and imaging technology, especially in the development of PET tracers.
Asia Pacific is expected to grow at the fastest CAGR growth of the radiopharmaceutical preclinical CRO market during the forecast period. The Asia-Pacific region includes a growing focus on research and development in developing nations and an increase in collaborations with pharmaceutical companies. This shift highlights the business and highlights the need for regionally tailored strategies to account for different research settings in this region.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the radiopharmaceutical preclinical CRO market along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Charles River Laboratories International, Inc.
- Pharmaron Beijing Co., Ltd.
- Novartis
- Cardinal Health, Inc.
- Bioscan, Inc.
- Jubilant Radiopharma
- Invicro (Konica Minolta)
- PerkinElmer, Inc.
- PAREXEL International Corporation
- Siemens Healthineers
- NorthStar Medical Radioisotopes, LLC
- IBA Radiopharma Solutions
- Eckert & Ziegler
- Covance, Inc. (Labcorp Drug Development)
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In March 2024, Novartis announced that it would pay $1 billion upfront to purchase Mariana Oncology, a radiopharmaceutical business, with the possibility of an additional $750 million in milestones. Mariana's pipeline in Massachusetts is headed by MC-339, a peptidic small molecule designed to deliver a payload of radioactive actinium and hailed as a potential treatment for small-cell lung cancer.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the radiopharmaceutical preclinical CRO market based on the below-mentioned segments:
Global Radiopharmaceutical Preclinical CRO Market, Radiopharmaceutical Type
- PET Tracers
- SPECT Tracers
- Therapeutic Radiopharmaceuticals
Global Radiopharmaceutical Preclinical CRO Market, By Therapeutic Area
- Oncology
- Cardiology
- Neurology
- Others
Frequently Asked Questions (FAQ)
-
1. What is the CAGR of the radiopharmaceutical preclinical CRO market over the forecast period?The radiopharmaceutical preclinical CRO market is projected to expand at a CAGR of 8.47% during the forecast period.
-
2. What is the market size of the radiopharmaceutical preclinical CRO market?The Global Radiopharmaceutical Preclinical CRO Market is Expected to Hold a Significant Share by 2033, at a CAGR of 8.47% during the forecast period 2023-2033.
-
3. Which region holds the largest share of the radiopharmaceutical preclinical CRO market?North America is anticipated to hold the largest share of the radiopharmaceutical preclinical CRO market over the predicted timeframe.
Need help to buy this report?