South Africa In Vitro Diagnostics Devices Market Size, Share, and COVID-19 Impact Analysis, By Technology (Clinical Chemistry, Immunoassay/Immunochemistry, Molecular Diagnostics, Hematology, and Microbiology), By Application (Oncology, Infectious Disease, Diabetes, Cardiology, and Nephrology), and South Africa In Vitro Diagnostics Devices Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareSouth Africa In Vitro Diagnostics Devices Market Insights Forecasts to 2033
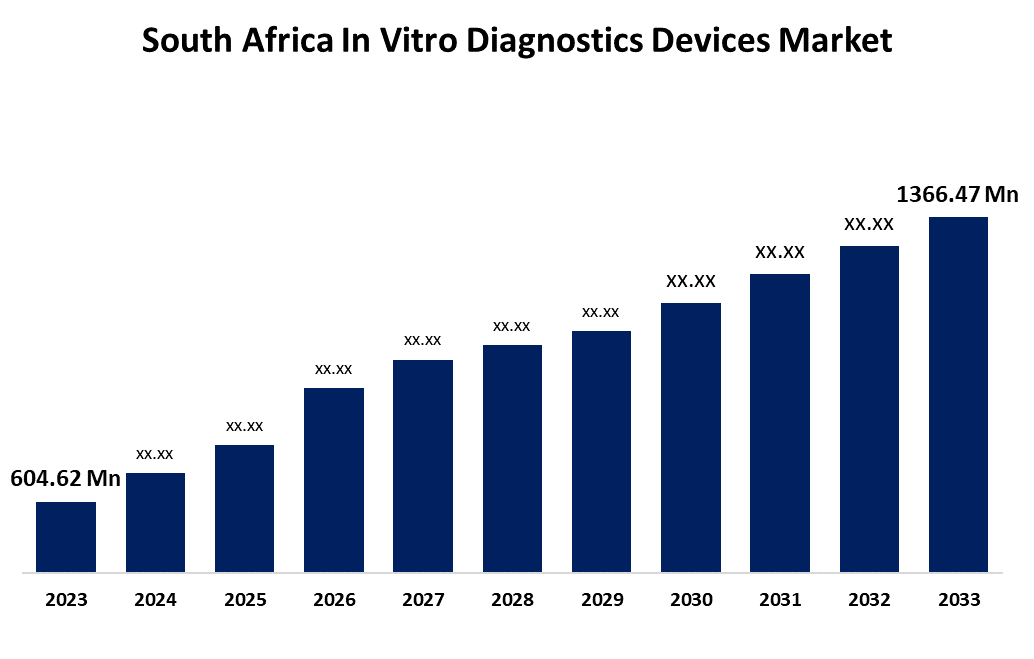
- The South Africa In Vitro Diagnostics Devices Market Size was valued at USD 604.62 Million in 2023.
- The Market Size is Growing at a CAGR of 8.50% from 2023 to 2033
- The South Africa In Vitro Diagnostics Devices Market Size is Expected to Reach USD 1366.47 Million by 2033
Get more details on this report -
The South Africa In Vitro Diagnostics Devices Market Size is Anticipated to Reach USD 1366.47 Million by 2033, Growing at a CAGR of 8.50% from 2023 to 2033
Market Overview
In vitro diagnostics devices represent tests on samples drawn from the human body, such as blood or tissue. Used to monitor general health, diseases, or other health conditions, and support treatment, in vitro diagnostic devices help identify which patients can be treated with the help of specific drugs or therapy. Next-generation sequencing tests are an example of IVDs that screen an individual's DNA to detect genomic variations. In vitro diagnostics are critical tools for everyday medical practice and emergencies, and numerous treatment decisions are based on their results. In this test, an analyte in a patient's sample is measured in person at the hospital rather than having it sent to a lab. This method can provide several advantages, such as stronger interventions and quicker outcomes. The market economy is anticipated to be significantly impacted by the development of condition-specific markers.
Report Coverage
This research report categorizes the South Africa in vitro diagnostics devices market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Africa in vitro diagnostics devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Africa in vitro diagnostics devices market.
South Africa In Vitro Diagnostics Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 604.62 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.50% |
| 2033 Value Projection: | USD 1366.47 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 191 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Technology, By Application |
| Companies covered:: | Abbott, Danaher, Siemens Healthineers AG, Roche Diagnostics, Sysmex Corporation, Beckman Coulter South Africa, Mindray South Africa, and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The rising chronically infectious diseases in the aged will increase the need for in vitro diagnostics in South Africa because these diseases are expected to positively target this high age category compared to the general population. Infectious diseases, including HIV, diabetes, cancers, and heart diseases, are known to constantly need monitoring and diagnosis, which is likely to increase the in vitro diagnostics market due to frequent cases of these and many other related diseases. In vitro diagnostics tests are key tools for identifying, diagnosing, and controlling these illnesses, creating opportunities for the in vitro diagnostics market to grow. Further, the increasing geriatric population in South Africa, along with expanding life expectancy, would fuel demand for in vitro diagnostics products over the entire forecast period. As the population grows, more medical attention and diagnostic services are required, predicted to contribute to market growth.
Restraining Factors
The high cost of innovative technologies in the in vitro diagnostics device market is expected to limit the growth of the market and discourage adoption among customers in South Africa, making molecular diagnostics, next-generation sequencing, and genetic testing out of reach for most small and medium-sized laboratories. Strict regulatory rules and skilled personnel could also increase the price tag.
Market Segmentation
The South Africa in vitro diagnostics devices market share is classified into technology and application.
- The molecular diagnostics segment is expected to hold the largest market share through the forecast period.
The South Africa in vitro diagnostics devices market is segmented by technology into clinical chemistry, immunoassay/immunochemistry, molecular diagnostics, hematology, and microbiology segments. Among these, the molecular diagnostics segment is expected to hold the largest market share through the forecast period. The major growth factors for the segment are growing infection cases, increased consumption of molecular diagnostic tests, and higher developments in the molecular diagnostics sector driven by the increasing prevalence of infectious diseases, such as HIV and influenza, in the country, more molecular diagnostic testing will occur. Innovative technologies from the new molecular diagnostics market spur growth in the industry. Factors that would give rise to increasing rates of infections of such diseases as HIV and influenza or new technologies in the sector of molecular diagnosis could thus be drivers for this expansion.
- The infectious disease segment is expected to dominate the South Africa in vitro diagnostics devices market during the forecast period.
Based on the application, the South Africa in vitro diagnostics devices market is divided into oncology, infectious disease, diabetes, cardiology, and nephrology. Among these, the infectious disease segment is expected to dominate the South Africa in vitro diagnostics devices market during the forecast period. It has contributed to the rise of infectious diseases, multidrug-resistant infections, and increasing demands for valuable diagnostic tools for such diseases.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Africa in vitro diagnostics devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott
- Danaher
- Siemens Healthineers AG
- Roche Diagnostics
- Sysmex Corporation
- Beckman Coulter South Africa
- Mindray South Africa
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Africa, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the South Africa in vitro diagnostics devices market based on the below-mentioned segments:
South Africa In Vitro Diagnostics Devices Market, By Technology
- Clinical Chemistry
- Immunoassay/Immunochemistry
- Molecular Diagnostics
- Hematology
- Microbiology
South Africa In Vitro Diagnostics Devices Market, By Application
- Oncology
- Infectious Disease
- Diabetes
- Cardiology
- Nephrology
Need help to buy this report?