South Korea In Vitro Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Clinical chemistry, Haematology, Immunodiagnostics, Molecular Diagnostics, and Others), By Product (Reagents, Instruments, and Others), By Application (Cardiology, Diabetes, Oncology, Infectious diseases, Nephrology, Autoimmune disease, and Others) By End User (Hospitals and Clinics, Diagnostics Laboratories, and Others), and South Korea In Vitro Diagnostics Market Insights Forecasts 2022 – 2032
Industry: HealthcareSouth Korea In Vitro Diagnostics Market Insights Forecasts to 2032
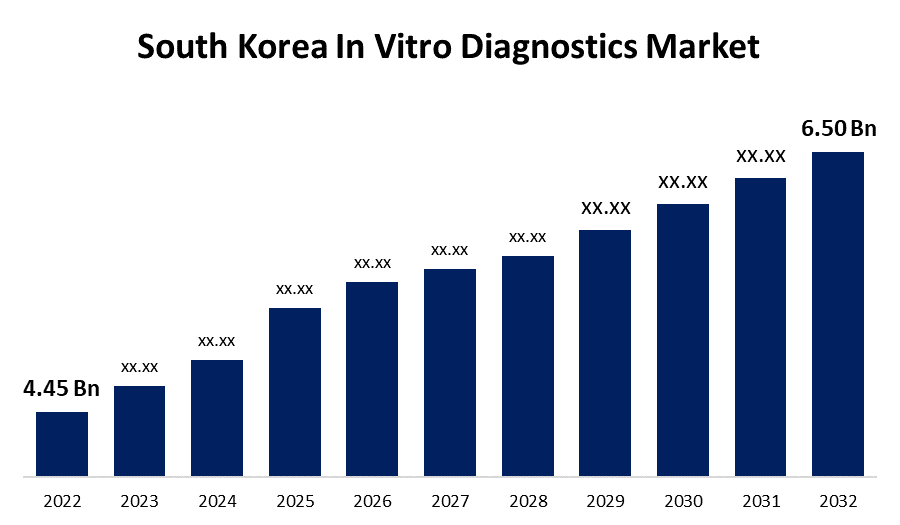
- The South Korean In Vitro Diagnostics Market Size was valued at USD 4.45 Billion in 2022.
- The Market Size is Growing at a CAGR of 3.86% from 2022 to 2032.
- The South Korea In Vitro Diagnostics Market Size is Expected To Reach 6.50 Billion by 2032.
Get more details on this report -
The South Korean In Vitro Diagnostics Market Size is Expected To Reach USD 6.50 Billion by 2032, at a CAGR of 3.86% during the forecast period 2022 to 2032.
The increased frequency of infectious diseases and chronic diseases, as well as the rise in older people, is a critical factor driving market growth. Additionally, the increased demand for fully automated instrumentation in laboratories is fueling the growth of the South Korea In Vitro Diagnostics Market.
Market Overview
In Vitro Diagnostics (IVD) are tests performed on samples taken from the human body, such as blood or tissue. In Vitro, diagnostics can detect diseases or other conditions and can be used to track a person's overall health to aid in disease treatment. Precision medicine may also make use of in vitro diagnostics to identify patients who are likely to benefit from specific treatments or therapies. Next-generation sequencing tests, which screen a person's DNA for genomic variations, are examples of in vitro diagnostics. In Vitro, diagnostics are important tools in medical practice and emergencies since they improve health outcomes. The majority of treatment decisions are now based on IVD results. The development and utilization of laboratory capacity are critical for providing effective healthcare to the people of South Korea. In vitro diagnostic point-of-care testing allows for the direct measurement of an analyte in a sample taken from a hospital patient without sending it to a laboratory. IVDPCT may have some advantages. Taking the sample and providing a result takes less time, which allows for more robust interventions, faster patient management, and better access to services.
Report Coverage
This research report categorizes the market for South Korean in vitro diagnostics market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korean in vitro diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Korean in vitro diagnostics market.
South Korea In Vitro Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 4.45 Billion |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 3.86% |
| 2032 Value Projection: | USD 6.50 Billion |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Test Type, By Product, By Application, By End User, and COVID-19 Impact Analysis. |
| Companies covered:: | Abbott Laboratories, Becton, Dickinson, and Company, Bio-Rad Laboratories Inc., Danaher Corporation, F. Hoffmann-La Roche AG, Siemens Healthineers, Sysmex Corporation, Thermo Fisher Scientific Inc., Seegene Inc., Gencurix, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Infectious and chronic disease outbreaks are expected to drive market growth during the forecast period. Rapid growth in the healthcare industry is a key factor driving market expansion. Additionally, R&D investment and technological advancement associated with in vitro diagnostics products and software are expected to provide significant opportunities for market participants.
Restraining Factors
Stringent government rules and regulations pose a significant challenge to market growth. The high cost of in vitro diagnostics test products is expected to limit market growth in the coming years.
Market Segment
- In 2022, the immunodiagnostics segment accounted for the largest revenue share over the forecast period.
Based on the test type, the South Korean In vitro diagnostics market is segmented into clinical chemistry, hematology, immuno diagnostics, molecular diagnostics, and others. Among these, the immunodiagnostics segment has the largest revenue share over the forecast period. Toxin detection is widely used in detecting infectious microbes. Immunodiagnostics is extremely accurate at detecting diseases. Since these tests are portable and can be used in low-resource settings, they are useful in a variety of healthcare settings. They are also well-known for their technical simplicity, speed, and adaptability, which makes them simple to use and implement in clinical laboratories.
- In 2022, the reagents segment accounted for the largest revenue share over the forecast period.
Based on product, the South Korea in vitro diagnostics market is segmented into reagents, instruments, and others. Among these, the reagents segment has the largest revenue share over the forecast period. The extensive R&D initiatives being undertaken by major market players for the development of novel reagents. The introduction of kits that allow for faster cancer detection allows companies to focus on niche profitable areas of the IVD business.
- In 2022, the infectious diseases segment accounted for the largest revenue share over the forecast period.
Based on application, the South Korea in vitro diagnostics market is segmented into cardiology, diabetes, oncology, infectious diseases, nephrology, autoimmune disease, and others. Among these, the infectious diseases segment has the largest revenue share over the forecast period. Due to rising incidences of pneumonia, hepatitis, tuberculosis, HIV-AIDS, and other infectious diseases in South Korea, as well as the growing demand for practical diagnostic tools for these diseases.
- In 2022, the hospitals and clinics segment accounted for the largest revenue share over the forecast period.
Based on end users, the South Korean in vitro diagnostics market is segmented into hospitals and clinics, diagnostics laboratories, and others. Among these, the hospitals and clinics segment has the largest revenue share over the forecast period. The increased bulk of diagnostic tests performed in hospitals, as well as the demand for hospital-based IVD tests, can be attributed to the large amount of diagnostic testing required for supporting clinical decisions and enhancing patient recovery. The majority of IVD devices are purchased by hospitals and used in large quantities.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea in vitro diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- Becton, Dickinson, and Company
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- F. Hoffmann-La Roche AG
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Seegene Inc.
- Gencurix
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In October 2022, Roche launched '2.0' antigen and antibody tests for COVID-19 in partnership with the South Korea-based SD Biosensor.
- In June 2022, the diagnostic reagent for monkeypox, "NovaplexTM MPXV Assay," was developed by Seegene, a South Korean molecular diagnosis company.
Market Segment
This study forecasts revenue at regional, and country levels from 2021 to 2032. Spherical Insights has segmented the South Korean In Vitro Diagnostics Market based on the below-mentioned segments:
South Korean In Vitro Diagnostics Market, By Test Type
- Clinical chemistry
- Hematology
- Immuno diagnostics
- Molecular Diagnostic
- Others
South Korea In Vitro Diagnostics Market, By Product
- Reagents
- Instruments
- Others
South Korea In Vitro Diagnostics Market, By Application
- Cardiology
- Diabetes
- Oncology
- Infectious diseases
- Nephrology
- Autoimmune disease
- Others
South Korea In Vitro Diagnostics Market, By End User
- Hospitals and Clinics
- Diagnostics Laboratories
- Others
Need help to buy this report?