Spain Liver Cancer Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Laboratory Tests, Imaging, Endoscopy, Biopsy, and Others), By End-Use (Hospitals & Diagnostic Laboratories, Academic & Research Institutes, and Pharmaceutical & CRO Laboratories), and Spain Liver Cancer Diagnostics Market Insights, Industry Trend, Forecasts to 2033.
Industry: HealthcareSpain Liver Cancer Diagnostics Market Insights Forecasts to 2033
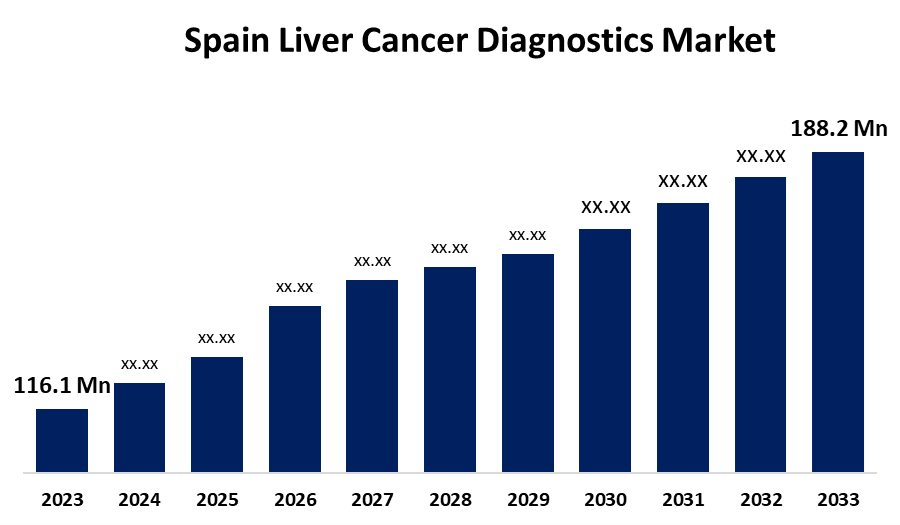
- The Spain Liver Cancer Diagnostics Market Size was valued at USD 116.1 Million in 2023.
- The Market Size is Growing at a CAGR of 4.95% from 2023 to 2033
- The Spain Liver Cancer Diagnostics Market Size is Expected to Reach USD 188.2 Million by 2033
Get more details on this report -
The Spain Liver Cancer Diagnostics Market Size is Anticipated to Reach USD 188.2 Million by 2033, growing at a CAGR of 4.95% from 2023 to 2033
Market Overview
Diagnostics refers to the identification of a disease or condition based on a patient’s signs and symptoms, as well as the methods employed to reach a diagnosis. Liver cancer diagnosis involves numerous tests that assess both the liver and blood. Each year, approximately 6,000 new liver cancer cases are documented in Spain, with a higher prevalence in men. The most frequently occurring primary liver tumor in Spain is hepatocellular carcinoma. Although cholangiocarcinoma is less prevalent, it constitutes at least 10% of primary liver cancers. Patients with liver cancer in Spain have a median survival time of 3.3 months, with possible extensions of up to 14.5 months for those with smaller tumors and maintained liver function. In 2022, 5,209 individuals in Spain succumbed to malignant liver tumors and intrahepatic bile duct cancers. Additionally, the growing occurrence of risk factors such as hepatitis B and C infections, excessive alcohol intake, and obesity serves as a significant driving force. There are opportunities for developing comprehensive screening initiatives and diagnostic instruments to identify liver cancer at earlier stages, enhancing patient outcomes and reducing mortality rates. Ongoing advancements in imaging technologies like MRI, CT scans, and ultrasound present opportunities to improve diagnostic precision. Merging artificial intelligence and machine learning techniques can further enhance diagnostic accuracy, facilitating early detection and effective treatment planning, ultimately bettering patient care and prognosis.
Report Coverage
This research report categorizes the market for the Spain liver cancer diagnostics based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Spain liver cancer diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Spain liver cancer diagnostics market.
Spain Liver Cancer Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 116.1 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 4.95% |
| 2033 Value Projection: | USD 188.2 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Test Type, By End-Use |
| Companies covered:: | Quibim, The Hospital Clínic-IDIBAPS, Roche Diagnostics, Vall d’Hebron Instituto de Oncología, IBEC, Institute for Bioengineering of Catalonia, IDIBELL, Fundación Profesor Novoa Santos, evitria, And Other Key Vendors |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis |
Get more details on this report -
Driving Factors
The liver cancer diagnostics market is influenced by various factors, including the rise in liver cancer cases in Spain and increasing liver cancer risk factors. There is a growing need for non-invasive, precise, and dependable diagnostic tests to facilitate earlier cancer detection. New diagnostic tools are consistently being introduced into the market. Machine learning has the potential to transform early cancer detection by teaching computers to recognize patterns in complex data. Additionally, the move toward personalized medicine has led to the creation and utilization of computer-based tests for diagnosing liver cancer. There are chances to discover new biomarkers that can reliably indicate disease progression, response to treatment, and recurrence, enabling tailored treatment approaches and enhancing patient outcomes.
Restraining Factors
Factors that hinder the growth of the liver cancer diagnostics market in Spain encompass a lack of awareness regarding early cancer symptoms, which may cause delayed diagnoses and subsequent poor prognoses. Hospitals in developing regions might struggle to afford the necessary diagnostic imaging equipment.
Market Segmentation
The Spain liver cancer diagnostics market share is classified into test type and end-use.
- The laboratory tests segment is expected to hold the largest market share through the forecast period.
The Spain liver cancer diagnostics market is segmented by test type into laboratory tests, imaging, endoscopy, biopsy, and others. Among these, the laboratory tests segment is expected to hold the largest market share through the forecast period. Several factors contribute to the high preference among the population for liver cancer diagnostics, notably due to their accuracy and cost-effectiveness. Laboratory tests primarily screen high-risk patients, aiding in determining various treatment options. These tests also play a crucial role in further assessing treatment plans and how they may impact other organs.
- The hospitals & diagnostic laboratories segment is expected to dominate the Spain liver cancer diagnostics market during the forecast period.
Based on the end-use, the Spain liver cancer diagnostics market is divided into hospitals & diagnostic laboratories, academic & research institutes, and pharmaceutical & CRO laboratories. Among these, the hospitals & diagnostic laboratories segment is expected to dominate the Spain liver cancer diagnostics market during the forecast period. Hospitals function as the primary centers for diagnosis and care for a large portion of the population. Conditions like Hepatocellular Carcinoma (HCC), cholangiocarcinoma, and others necessitate facilities capable of long-term disease management and treatment. Additionally, hospitals and diagnostic labs offer patients affordable screening solutions. The availability of skilled professionals, advanced methodologies, sample collection, and highly sensitive data in one location leads to a quick turnaround time. Consequently, these environments are highly sought after and contribute significantly to revenue generation.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Spain liver cancer diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Quibim
- The Hospital Clínic-IDIBAPS
- Roche Diagnostics
- Vall d'Hebron Instituto de Oncología
- IBEC, Institute for Bioengineering of Catalonia
- IDIBELL
- Fundación Profesor Novoa Santos
- evitria
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In March 2024, Quibim, a leading global company focused on imaging biomarkers for precision medicine, has introduced a new product, QP-Liver, designed to enhance the diagnosis of diffuse liver diseases through precise quantification of tissue fat and iron from MRI scans. This product has received CE and UKCA certifications for the European Union, including Spain and the United Kingdom, indicating its approval for use in these markets.
Market Segment
This study forecasts revenue at Spain, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Spain Liver Cancer Diagnostics Market based on the below-mentioned segments:
Spain Liver Cancer Diagnostics Market, By Test Type
- Laboratory Tests
- Imaging
- Endoscopy
- Biopsy
- Others
Spain Liver Cancer Diagnostics Market, By End-Use
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Pharmaceutical & CRO Laboratories
Need help to buy this report?