United Kingdom In-Vitro Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (Instruments, Reagents, and Others), By Technology (Immunoassay, Hematology, Clinical Chemistry, Molecular Diagnostics, Coagulation, Microbiology, and Others), By Application (Infectious Diseases, Diabetes, Oncology, Cardiology, Nephrology, Autoimmune Diseases, Drug Testing, and Others), and United Kingdom In-Vitro Diagnostics Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareUnited Kingdom In-Vitro Diagnostics Market Insights Forecasts to 2033
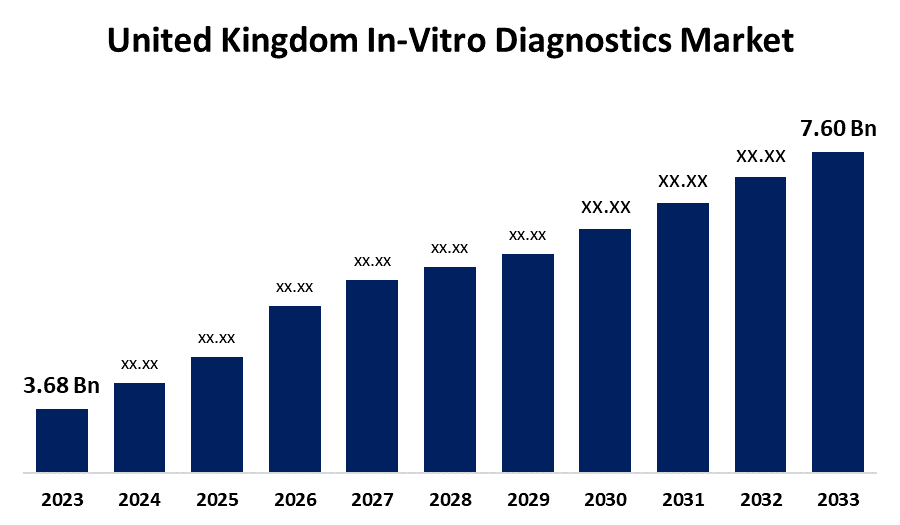
- The U.K. In-Vitro Diagnostics Market Size was valued at USD 3.68 Billion in 2023.
- The U.K. In-Vitro Diagnostics Market Size is growing at a CAGR of 7.52% from 2023 to 2033
- The U.K. In-Vitro Diagnostics Market Size is expected to reach USD 7.60 Billion by 2033
Get more details on this report -
The United Kingdom In-Vitro Diagnostics Market Size is anticipated to exceed USD 7.60 Billion by 2033, growing at a CAGR of 7.52% from 2023 to 2033. The growing prevalence of chronic diseases, awareness & adoption of personalized medicine, and rising use of point-of-care (POC) diagnostics are driving the growth of the in-vitro diagnostics market in the UK.
Market Overview
In-vitro diagnostics market is the industry comprising of medical devices and tests performed outside the body, using biological samples like blood or urine for detecting diseases, monitoring conditions, and assess predispositions. In-vitro diagnostics aids in patient diagnosis and treatment. In-vitro diagnostic tests are typically conducted in test tubes and similar equipment, as opposed to in vivo tests in which test is conducted in the body itself. The test includes vast area from complex technologies used in clinical laboratories to simple, patient friendly quick testing kits. The trend of personalized medicine and the need for in vitro diagnostic testing are escalating the IVD market. The increasing cases of chronic & infectious diseases, advancements in diagnostic technologies, and shift towards personalized medicine and point-of care testing are all the factors that are escalating the lucrative market growth opportunities for in-vitro diagnostics.
Report Coverage
This research report categorizes the market for the UK in-vitro diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United Kingdom in-vitro diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the UK in-vitro diagnostics market.
United Kingdom In-Vitro Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 3.68 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 7.52% |
| 2033 Value Projection: | USD 7.60 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Product, By Technology, By Application |
| Companies covered:: | Abbott, Becton, Dickinson and Company, BioMerieux SA, Bio-Rad Laboratories, Inc., Danaher Corporation, F. Hoffmann-La Roche Ltd, QIAGEN, Siemens Healthineers AG, Sysmex Corporation, Thermo Fisher Scientific Inc., Fujifilm Holdings Corporation, and Others |
| Pitfalls & Challenges: | COVID-19 impact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The growing prevalence of chronic diseases especially allergy conditions, high blood pressure, low back disorder and depression among men and women drives the market demand. IVD tests enables medical professionals to devise more personalized treatments, based on faster, more precise diagnoses. Nearly 70% of clinical decisions rely on such tests. The awareness and adoption of personalized medicine contributing to propel the IVD market as many IVD tests are benefitting from the adoption of personalized medicine. The increasing use of point-of-care diagnostics testing in IVD drives the market for in-vitro diagnostics as it enables faster results and quicker decision-making for clinicians, ultimately supporting timely diagnosis, monitoring, and treatment.
Restraining Factors
The strict regulations associated with in-vitro diagnostic (IVD) for ensuring patient safety and improving patient outcome is challenging the market growth.
Market Segmentation
The United Kingdom in-vitro diagnostics market share is classified into product, technology, and application.
- The reagents segment dominated the market with the largest market share in 2023 and is expected to grow at a significant CAGR during the projected period.
The United Kingdom in-vitro diagnostics market is segmented by product into instruments, reagents, and others. Among these, the reagents segment dominated the market with the largest market share in 2023 and is expected to grow at a significant CAGR during the projected period. Clinical hematology and body fluid testing reagents, clinical chemistry reagents, clinical immunology reagents, and microbiology, cell histology and molecular biology reagents are some of the examples of in-vitro diagnostics reagents.
- The immunoassay segment held the largest share of the UK in-vitro diagnostics market in 2023 and is expected to grow at a significant CAGR during the projected period.
The United Kingdom in-vitro diagnostics market is segmented by technology into immunoassay, hematology, clinical chemistry, molecular diagnostics, coagulation, microbiology, and others. Among these, the immunoassay segment held the largest share of the UK in-vitro diagnostics market in 2023 and is expected to grow at a significant CAGR during the projected period. Immunoassay commonly used for testing infectious diseases and other biomarkers. The technology rely on the specific interaction between antibodies and antigens, detecting and measuring substances such as hormones, proteins, and antibodies.
- The infectious diseases segment dominated the UK in-vitro diagnostics market in 2023 and is expected to grow at a significant CAGR during the projected period.
The United Kingdom in-vitro diagnostics market is segmented by application into infectious diseases, diabetes, oncology, cardiology, nephrology, autoimmune diseases, drug testing, and others. Among these, the infectious diseases segment dominated the UK in-vitro diagnostics market in 2023 and is expected to grow at a significant CAGR during the projected period. IVD aid in managing RSV, reducing the magnitude of infection within a community by limiting infectious interactions through timely PoC detection and diagnosis.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the U.K. in-vitro diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott
- Becton, Dickinson and Company
- BioMerieux SA
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- F. Hoffmann-La Roche Ltd
- QIAGEN
- Siemens Healthineers AG
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Fujifilm Holdings Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In August 2024, Eurobio Scientific, a leading French group in in vitro specialty medical diagnostics and life sciences, announced the finalisation of its agreement with Myriad Genetics to acquire the EndoPredict genomic test.
- In May 2024, Prolight Diagnostics has been awarded a SEK 17 million grant from National Institute for Health and Care Research’s i4i Product Development Awards, supporting the development of the Psyros point-of-care test for heart attack.
Market Segment
This study forecasts revenue at U.K., regional, and country levels from 2020 to 2033. Spherical Insights has segmented the United Kingdom in-vitro diagnostics Market based on the below-mentioned segments:
UK In-Vitro Diagnostics Market, By Product
- Instruments
- Reagents
- Others
UK In-Vitro Diagnostics Market, By Technology
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostics
- Coagulation
- Microbiology
- Others
UK In-Vitro Diagnostics Market, By Application
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Diseases
- Drug Testing
- Others
Need help to buy this report?