United Kingdom Pharmacovigilance Market Size, Share, and COVID-19 Impact Analysis, By Service Provider (In-house and Contract Outsourcing), By Product Life Cycle (Pre-clinical, Phase I, Phase II, Phase III, and Phase IV), By Type (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, and EHR Mining), By Process Flow (Case Data Management, Signal Detection, and Risk Management System) By Therapeutic Area (Oncology, Neurology, Cardiology, respiratory Systems, and Others), By End-use (Pharmaceuticals, Biotechnology Companies, Medical Device Manufacturers, and Others), and United Kingdom Pharmacovigilance Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareUnited Kingdom Pharmacovigilance Market Insights Forecasts to 2033
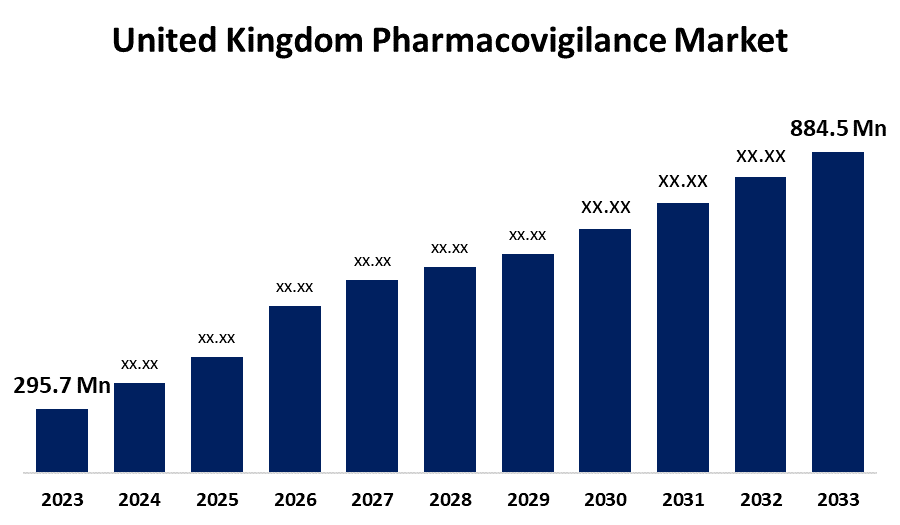
- The U.K. Pharmacovigilance Market Size was valued at USD 295.7 Million in 2023.
- The Market Size is Growing at a CAGR of 11.58% from 2023 to 2033
- The U.K. Pharmacovigilance Market Size is Expected to reach USD 884.5 Million by 2033
Get more details on this report -
The United Kingdom Pharmacovigilance Market Size is Anticipated to Exceed USD 884.5 Million by 2033, Growing at a CAGR of 11.58% from 2023 to 2033. The Growing cases of ADRs, increasing production of new drugs, advancement in ADR database & information systems, and stringent regulatory framework are driving the growth of the pharmacovigilance market in the UK.
Market Overview
Pharmacovigilance is the process and science of monitoring the safety of medicines and taking action to reduce the adverse effects/ risks associated with the medicines and increase their benefits. According to WHO, pharmacovigilance is related to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine-related problem. It is essential to enhance public health outcomes and directing regulatory choices to reduce medication-related damage. The increase in cases of adverse drug reactions (ADRs) has surged the need for services and solutions to efficiently track, evaluate, and manage drug safety data. The integration of AI in PV significantly accelerates the efficiency and effectiveness of drug safety monitoring processes, which ultimately aids in improving the capability to detect and asses ADR. Thus, AI helps regulatory bodies, PV specialists, and pharmaceutical businesses make more educated decisions as it enhances the capacity to identify and evaluate ADRs.
Report Coverage
This research report categorizes the market for the UK pharmacovigilance market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United Kingdom pharmacovigilance market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the UK pharmacovigilance market.
United Kingdom Pharmacovigilance Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 295.7 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 11.58% |
| 2033 Value Projection: | USD 884.5 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 191 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Service, By Type, By Process Flow, By Therapeutic Area, By End-use |
| Companies covered:: | Accenture, Cognizant, IBM Corporation, IQVIA, ArisGlobal, ICON plc., Capgemini, PAREXEL International Corporation, TAKE Solutions Ltd., BioClinica Inc., and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The prevalence of disorders that require a combination of pharmaceuticals as well as the rising incidence of Adverse Drug Reactions (ADRs) due to drug addiction are driving up the UK pharmacovigilance market. Further, the increase in the production of new pharmaceuticals and the existence of strict government regulations pertaining to drug safety are responsible for propelling the market growth. Developments in ADR databases and information systems have made it possible to report correct data, which research experts may then use for potential clinical trials. The growing number of R&D activities and new drug development along with the adaptive trial designs are anticipated to drive the market.
Restraining Factors
The inconsistent adverse event reporting in low and middle-income nations is responsible for restraining the pharmacovigilance market.
Market Segmentation
The United Kingdom Pharmacovigilance Market share is classified into service provider, product life cycle, type, process flow, therapeutic area, and end-use.
- The contract outsourcing segment accounted for the largest market share in 2023.
The United Kingdom pharmacovigilance market is segmented by service provider into in-house and contract outsourcing. Among these, the contract outsourcing segment accounted for the largest market share in 2023. Contract outsourcing promotes resource flexibility, secures additional flexibility, secures additional capacity, and reduces the need for large investments as well as fixed overhead expenses. The growing number of biotechnology and pharmaceutical companies outsourcing their PV operations to service providers including contract research organizations are driving the market growth.
- The phase IV segment dominates the market with the largest market share in 2023.
The United Kingdom pharmacovigilance market is segmented by product life cycle into pre-clinical, phase I, phase II, phase III, and phase IV. Among these, the phase IV segment dominates the market with the largest market share in 2023. Phase IV studies monitor and evaluate adverse events, drug interactions, and other drug-related hazards, supporting pharmacovigilance activities that contribute to the updating of safety data, the improvement of prescribing guidelines, and the protection of patients during broad use. The increased R&D activities and clinical research along with the top class research funders and pharmaceutical industries in the region are promoting the market growth.
- The spontaneous reporting segment held the largest revenue share of the UK pharmacovigilance market in 2023.
Based on the type, the U.K. pharmacovigilance market is divided into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring, and EHR mining. Among these, the spontaneous reporting segment held the largest revenue share of the UK pharmacovigilance market in 2023. Spontaneous reporting integrated with targeted and active reporting provides early safety signals of unknown medicine-related responses. The higher drug safety data provided by spontaneous reporting during the post-marketing phase than clinical trials during medication development is responsible for driving the market demand in the spontaneous reporting segment.
- The signal detection segment dominated the UK pharmacovigilance market in 2023.
The United Kingdom pharmacovigilance market is segmented by process flow into case data management, signal detection, and risk management system. Among these, the signal detection segment dominated the UK pharmacovigilance market in 2023. Pharmacovigilance is seeing an increase in the significance of signal detection and management. The increasing need for the methodical and controlled process of signal management is promoting market growth. The regulatory authority is increasingly looking for Marketing Authorization Holders to be able to establish a methodical and controlled process of signal management.
- The oncology segment is anticipated to grow at the fastest CAGR growth during the forecast period.
Based on the therapeutic area, the U.K. pharmacovigilance market is divided into oncology, neurology, cardiology, respiratory systems, and others. Among these, the oncology segment is anticipated to grow at the fastest CAGR growth during the forecast period. From 2015 to 2017, there were almost 1,000 new instances of cancer every day in the nation or about 367,000 new cases annually. Cancer Research U.K. selected Ideagen's Q-Pulse and PleaseReview products in June 2019. The rising prevalence of cancer surges the need for new drug development leading to increasing R&D and clinical research resulting in driving the market demand in the oncology segment.
- The pharmaceuticals segment dominates the UK pharmacovigilance market with the largest market share in 2023.
Based on the end-use, the U.K. pharmacovigilance market is divided into pharmaceuticals, biotechnology companies, medical device manufacturers, and others. Among these, the pharmaceuticals segment dominates the UK pharmacovigilance market with the largest market share in 2023. Pharmacovigilance groups have to manage risk while simultaneously keeping up with product innovation, technological advancements, and shifting regulatory requirements as the growing pharmaceutical industry encounters increasingly intricate and novel issues.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the U.K. pharmacovigilance market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Accenture
- Cognizant
- IBM Corporation
- IQVIA
- ArisGlobal
- ICON plc.
- Capgemini
- PAREXEL International Corporation
- TAKE Solutions Ltd.
- BioClinica Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In February 2022, Cognizant announced a partnership with Medable Inc. to jointly deliver clinical research solutions based on Medable’s software-as-a-service platform for decentralized clinical trials.
- In February 2022, Full-service contract research organisation (CRO) LINK Medical entered a collaboration agreement with Viedoc for a new partnership program to enhance clinical trial efficiency.
Market Segment
This study forecasts revenue at U.K., regional, and country levels from 2020 to 2033. Spherical Insights has segmented the United Kingdom Pharmacovigilance Market based on the below-mentioned segments:
UK Pharmacovigilance Market, By Service Provider
- In-house
- Contract Outsourcing
UK Pharmacovigilance Market, By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
UK Pharmacovigilance Market, By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
UK Pharmacovigilance Market, By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
UK Pharmacovigilance Market, By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
UK Pharmacovigilance Market, By End-use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
Need help to buy this report?