United States Capnography Devices Market Size, Share, and COVID-19 Impact Analysis, By Product (Portable and Stationary), By Solution Offering (Integrated and Standalone), By Parameter (Single Parameter and Multiparameter), By Technology (Mainstream, Sidestream, and Microstream), By Component (OEM Modules, Filters, Sensors, Sampling Line, and Other Accessories), By Application (Emergency Medicine, Pain Medicine, Procedural Sedation, Critical Care, and Others), By End Use (Hospitals, Ambulatory Care Centers, and Others), and United States Capnography Devices Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareUnited States Capnography Devices Market Insights Forecasts to 2033
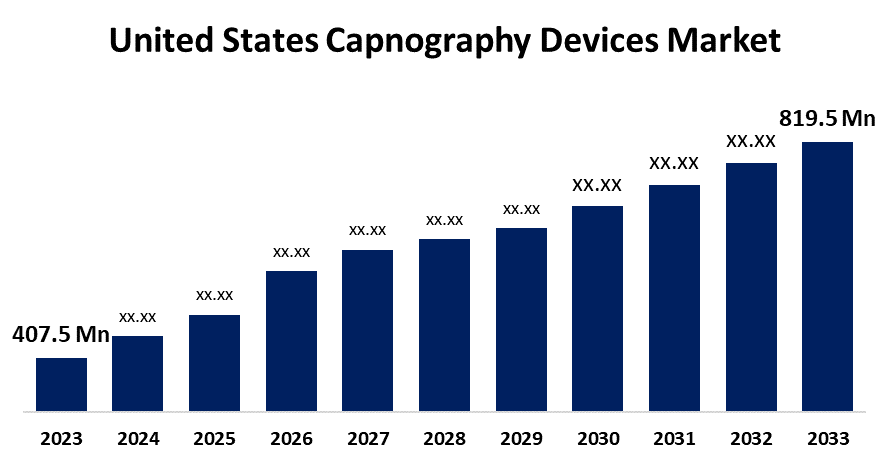
- The U.S. Capnography Devices Market Size was valued at USD 407.5 Million in 2023.
- The Market Size is Growing at a CAGR of 7.24% from 2023 to 2033
- The U.S. Capnography Devices Market Size is Expected to reach USD 819.5 Million by 2033
Get more details on this report -
The United States Capnography Devices Market Size is anticipated to Exceed USD 819.5 Million by 2033, Growing at a CAGR of 7.24% from 2023 to 2033. The Growing incidence of respiratory diseases and technological advancements are driving the Growth of the capnography devices market in the US.
Market Overview
Capnography devices are medical machines that are used to monitor the concentration or partial pressure of carbon dioxide in breathing gases. This medical gadget quickly assess respiratory health to help medical professionals act more quickly. It monitors the carbon dioxide (CO2) content of exhaled air and offers graphical waveform readings and real-time data. According to data from the US Environmental Protection Agency, in 2020 there were 369 emergency room visits for respiratory conditions overall and asthma specifically, accounting for 47 visits for asthma and 322 visits for other respiratory conditions for every 10,000 children. The need for capnography devices which are necessary for efficient monitoring and prompt intervention, better patient outcomes, and integration into standard treatment protocols is driven by the significant prevalence of respiratory disorders. The integration of capnography devices with other monitoring instruments, such as pulse oximeters is advantageous in clinical settings for monitoring respiratory status in patients. Developing artificial intelligence (AI) is a noteworthy trend in technical breakthroughs as it attempts to improve data interpretation and accuracy.
Report Coverage
This research report categorizes the market for the US capnography devices market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United States capnography devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the US capnography devices market.
United States Capnography Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 407.5 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 7.24% |
| 2033 Value Projection: | USD 819.5 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 191 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By Solution Offering, By Parameter, By Technology, By Component, By Application, By End Use |
| Companies covered:: | Infinium Medical, Inc., Masimo, Medtronic, Koninklijke Philips N.V., ICU Medical Inc., Nihon Kohden Corporation, Nonin, BD, Avante Health Solutions, EdanUSA, and Others |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
According to WHO, 38.7% of children with asthma who were 18 years of age or younger and 39.6% of adults ages 18 and older who had asthma in 2021 reported having experienced one or more asthma attacks in the previous year along with 4.9 million doctors' office visits. The increased prevalence of respiratory diseases in the US contributes to market demand. Due to the pandemic in 2020, Medtronic predicted increased demand for goods including ventilators, pulse oximeters, advanced parameter monitoring, extracorporeal life support, and capnography. The pandemic has positively influenced market growth. The developments in data analytics and sensor technologies with better patient monitoring and data management by the integration of modern capnography devices with electronic health records (EHRs) are driving market growth.
Restraining Factors
Strict regulations in the US may hamper the market for capnography devices as it takes three to seven years to demonstrate a new device's safety for medical use.
Market Segmentation
The United States Capnography Devices Market share is classified into product, solution offering, parameter, technology, component, application, and end use.
- The portable segment dominates the US capnography devices market with the largest share in 2023.
The United States capnography devices market is segmented by product into portable and stationary. Among these, the portable segment dominates the US capnography devices market with the largest share in 2023. Portable carnography devices are compact, easy to use, and deliver accurate results. Because of their portability and versatility, these devices enable medical professionals to keep an eye on patients' respiratory conditions in a variety of locations, including emergency response vehicles, outpatient clinics, and homes.
- The integrated segment dominates the US capnography devices market during the forecast period.
Based on the solution offering, the U.S. capnography devices market is divided into integrated and standalone. Among these, the integrated segment dominates the US capnography devices market during the forecast period. Capnography devices integrated with existing patient monitoring offer continuous patient monitoring along with enhanced workflow efficiency and reduced complexity in patient care management. The widespread adoption of integrated systems in different clinical settings to facilitate seamless patient management across a range of healthcare settings is driving the market.
- The multiparameter capnography segment accounted for the largest revenue share of the US capnography devices market in 2023.
The United States capnography devices market is segmented by parameter into single parameter and multiparameter. Among these, the multiparameter capnography segment accounted for the largest revenue share of the US capnography devices market in 2023. Multiparameter capnography offers thorough respiratory monitoring in addition to other metrics like blood pressure, heart rate, pulse oximetry, and more. These devices provide real-time data on many physiological indicators, streamlining clinical procedures and improving patient care, offering integrating monitoring capabilities. Their widespread adoption can be attributed to their capacity to combine several monitoring functions into a single device.
- The sidestream segment accounted for the largest revenue share of the US capnography devices market in 2023.
The United States capnography devices market is segmented by technology into mainstream, sidestream, and microstream. Among these, the sidestream segment accounted for the largest revenue share of the US capnography devices market in 2023. End-Tidal CO2 (EtCO2) levels can be continuously monitored with sidestream devices, which is beneficial for evaluating ventilation and respiratory status in both intubated and non-intubated patients. Further, their exceptional precision in identifying alterations in respiratory function enables timely intervention in critical care environments. The widespread adoption of sidestream devices from anesthesia to intensive care are driving the market.
- The sensors segment dominates the US capnography devices market during the forecast period.
Based on the component, the U.S. capnography devices market is divided into OEM modules, filters, sensors, sampling line, and other accessories. Among these, the sensors segment dominates the US capnography devices market during the forecast period. Sensors are crucial parts that have a direct impact on the dependability and efficiency of respiratory monitoring devices that use capnography. Advances in sensor technology have increased sensor durability, accuracy, and compatibility with different patient monitoring systems. Their vital significance in precisely measuring and capturing end-tidal carbon dioxide (EtCO2) levels is driving the market growth.
- The procedural sedation segment dominates the US capnography devices market with the largest revenue share in 2023.
Based on the application, the U.S. capnography devices market is divided into emergency medicine, pain medicine, procedural sedation, critical care, and others. Among these, the procedural sedation segment dominates the US capnography devices market with the largest revenue share in 2023. Capnography helps monitor breathing rate, patterns, and airway patency under milder sedation. When utilizing tracheal tubes and airway devices, especially in situations where there is a reduced reaction to vocal stimuli, waveform capnography monitoring is advised during procedural sedation. According to the American Society of Anaesthesiologists' standards, patients in moderate to deep sedation must be monitored using visual observation, and capnography.
- The hospitals segment dominates the market with the largest market share during the forecast period.
The United States capnography devices market is segmented by end use into hospitals, ambulatory care centers, and others. Among these, the hospitals segment dominates the market with the largest market share during the forecast period. Capnography equipment is necessary in hospitals for several purposes, such as monitoring anesthesia, emergency rooms, intensive care units (ICUs), procedural sedation, and general patient monitoring. The real-time respiratory status monitoring and the early detection of respiratory compromise drive the market demand in the hospitals segment.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the U.S. capnography devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Infinium Medical, Inc.
- Masimo
- Medtronic
- Koninklijke Philips N.V.
- ICU Medical Inc.
- Nihon Kohden Corporation
- Nonin
- BD
- Avante Health Solutions
- EdanUSA
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In June 2023, Royal Philips, a global leader in health technology, and Masimo, a global medical technology company, announced FDA clearance allowing the activation of SedLine Brain Function Monitoring, Regional Oximetry, and Carbon dioxide measurements in Philips Patient Monitors – IntelliVue MX750 and MX850.
- In June 2022, GE Healthcare, Medtronic received FDA 510(k) clearance and CE Mark approval on the integration of advanced INVOS regional oximetry and Microstream capnography technologies on the CARESCAPE precision monitoring platform.
Market Segment
This study forecasts revenue at U.S., regional, and country levels from 2020 to 2033. Spherical Insights has segmented the United States Capnography Devices Market based on the below-mentioned segments:
US Capnography Devices Market, By Product
- Portable
- Stationary
US Capnography Devices Market, By Solution Offering
- Integrated
- Standalone
US Capnography Devices Market, By Parameter
- Single Parameter
- Multiparameter
US Capnography Devices Market, By Technology
- Mainstream
- Sidestream
- Microstream
US Capnography Devices Market, By Component
- OEM Modules
- Filters
- Sensors
- Sampling Line
- Other Accessories
US Capnography Devices Market, By Application
- Emergency Medicine
- Pain Medicine
- Procedural Sedation
- Critical Care
- Others
US Capnography Devices Market, By End Use
- Hospitals
- Ambulatory Care Centers
- Others
Need help to buy this report?