US PTA Balloon Catheter Market Size, Share, and COVID-19 Impact Analysis, By Material Type (Polyurethane and Nylon), By Application (Peripheral Artery Disease and Coronary Artery Disease), By End User (Hospitals and Ambulatory Surgical Centers), and US PTA Balloon Catheter Market Insights, Industry Trend, Forecasts to 2033.
Industry: HealthcareUS PTA Balloon Catheter Market Insights Forecasts to 2033
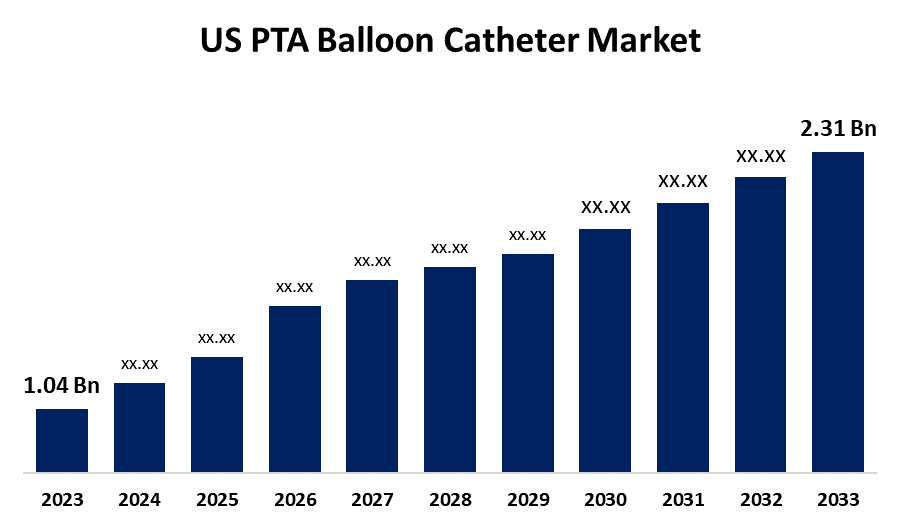
- The United States PTA Balloon Catheter Market Size was valued at USD 1.04 Billion in 2023.
- The Market is Growing at a CAGR of 8.31% from 2023 to 2033
- The U. S. PTA Balloon Catheter Market Size is Expected to reach USD 2.31 Billion by 2033
Get more details on this report -
US PTA Balloon Catheter Market Size is anticipated to Exceed USD 2.31 Billion by 2033, Growing at a CAGR of 8.31% from 2023 to 2033.
Market Overview
PTA balloons differ in length, width, and form based on the anatomy they are meant to treat. Percutaneous transluminal angioplasty (PTA) is the acronym for the procedure (percutaneous meaning through the skin). An inflatable balloon is utilized at the tip of a PTA balloon catheter during a minimally invasive catheterization technique. The goal of this operation is to widen a vessel's opening. The deflated balloon is placed in the constricted area, inflated briefly, and then deflated once more to be taken out. PTA balloons vary in length, width, and form based on the anatomy they are meant to treat. There are two methods for treating peripheral vascular lesions with PTA balloon catheters. Extending the lumen of blocked blood arteries is one method. The term "plain-old balloon angioplasty" (POBA) describes this technique. Expanding stents to treat vascular obstruction is the second application. An aging population, the rising use of minimally invasive procedures, and the incidence of cardiovascular illnesses are all important drivers of market expansion. The American Heart Association reports that heart disease is the greatest cause of death in the country for people of all ages, genders, and races.
Report Coverage
This research report categorizes the market for the US PTA balloon catheter market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the PTA balloon catheter market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the PTA balloon catheter market.
US PTA Balloon Catheter Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 1.04 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 8.31% |
| 2033 Value Projection: | USD 2.31 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 199 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Material Type, By Application, By End User |
| Companies covered:: | Abbott, Terumo Corporation, Boston Scientific Corporation, Cook Group, Biotronik, PAN Medical US Corporation, Cardinal Health, Medtronic, Cagent Vascular, and Others |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The rise of cardiovascular ailments, the aging population, and the growing usage of minimally invasive procedures are all significant factors driving market expansion. According to the American Heart Association, heart disease is the leading cause of mortality in the nation for individuals of all racial, gender, and age groups. Moreover, the aging population's higher risk of cardiovascular disease is another factor fueling this market's expansion. Furthermore, the development of more sophisticated PTA balloon catheters with better performance has been made possible by technological breakthroughs. PTA operations were safer and more successful thanks to these advances, which also included drug-coated balloons and state-of-the-art materials, spurring market expansion.
Restraining Factors
In the US, PTA balloon catheters are governed by the Food and Drug Administration. The US body in charge of regulating medical devices, the Food and Drug Administration (FDA), publishes guidelines about specifications for specific Class II uses. Therefore, it is anticipated that US product recalls might hinder market expansion.
Market Segmentation
The US PTA balloon catheter market share is classified into material type, application, and end user.
- The nylon segment is expected to hold the largest market share through the forecast period.
The US PTA balloon catheter market is segmented by material type into polyurethane and nylon. Among these, the nylon segment is expected to hold the largest market share through the forecast period. Because of its remarkable strength and durability, nylon is a highly favored material for medical equipment like balloon catheters.
- The peripheral artery disease segment dominates the market with the largest market share over the predicted period.
The US PTA balloon catheter market is segmented by application into peripheral artery disease and coronary artery disease. Among these, the peripheral artery disease segment dominates the market with the largest market share over the predicted period. By offering a secure and reliable remedy for arterial blockages in different areas of the peripheral vasculature, PTA balloon catheters are essential in the treatment of peripheral artery disease (PAD). These cutting-edge medical innovations greatly enhanced patient outcomes and increased the range of available therapies for PAD patients.
- The hospitals segment is expected to hold the largest share of the US PTA balloon catheter market during the forecast period.
Based on the end user, the US PTA balloon catheter market is divided into hospitals and ambulatory surgical centers. Among these, the hospitals segment is expected to hold the largest share of the US PTA balloon catheter market during the forecast period. This is explained by the rising incidence of cardiovascular disease, the expanding patient base, and the rise in cardiac implant operations.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the US PTA balloon catheter market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott
- Terumo Corporation
- Boston Scientific Corporation
- Cook Group
- Biotronik
- PAN Medical US Corporation
- Cardinal Health
- Medtronic
- Cagent Vascular
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In November 2023, The Preside hydrophilic medical device coating technology was introduced by Surmodics, Inc. This innovative coating technology is intended especially for the newest peripheral vascular, coronary, and neurovascular devices. It provides enhanced lubricity and coating durability.
Market Segment
This study forecasts revenue at US, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the US PTA Balloon Catheter Market based on the below-mentioned segments:
US PTA Balloon Catheter Market, By Material Type
- Polyurethane
- Nylon
US PTA Balloon Catheter Market, By Application
- Peripheral Artery Disease
- Coronary Artery Disease
US PTA Balloon Catheter Market, By End User
- Hospitals
- Ambulatory Surgical Centers
Need help to buy this report?